Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10464
Revised: February 9, 2014
Accepted: April 30, 2014
Published online: August 14, 2014
AIM: To evaluate the most cost-effectiveness strategy for preventing variceal growth and bleeding in patients with cirrhosis and small esophageal varices.
METHODS: A stochastic analysis based on decision trees was performed to compare the cost-effectiveness of beta-blockers therapy starting from a diagnosis of small varices (Strategy 1) with that of endoscopic surveillance followed by beta-blockers treatment when large varices are demonstrated (Strategy 2), for preventing variceal growth, bleeding and death in patients with cirrhosis and small esophageal varices. The basic nodes of the tree were gastrointestinal endoscopy, inpatient admission and treatment for bleeding, as required. All estimates were performed using a Monte Carlo microsimulation technique, consisting in simulating observations from known probability distributions depicted in the model. Eight-hundred-thousand simulations were performed to obtain the final estimates. All estimates were then subjected to Monte Carlo Probabilistic sensitivity analysis, to assess the impact of the variability of such estimates on the outcome distributions.
RESULTS: The event rate (considered as progression of varices or bleeding or death) in Strategy 1 [24.09% (95%CI: 14.89%-33.29%)] was significantly lower than in Strategy 2 [60.00% (95%CI: 48.91%-71.08%)]. The mean cost (up to the first event) associated with Strategy 1 [823 £ (95%CI: 106 £-2036 £)] was not significantly different from that of Strategy 2 [799 £ (95%CI: 0 £-3498 £)]. The cost-effectiveness ratio with respect to this endpoint was equal to 50.26 £ (95%CI: -504.37 £-604.89 £) per event avoided over the four-year follow-up. When bleeding episodes/deaths in subjects whose varices had grown were included, the mean cost associated with Strategy 1 was 1028 £ (95%CI: 122 £-2581 £), while 1699 £ (95%CI: 171 £-4674 £) in Strategy 2.
CONCLUSION: Beta-blocker therapy turn out to be more effective and less expensive than endoscopic surveillance for primary prophylaxis of bleeding in patients with cirrhosis and small varices.
Core tip: In patients with cirrhosis and small esophageal varices no study so far has evaluated the economical consequences of replacing traditional endoscopic surveillance with primary prophylaxis with beta-blockers from this stage. In this study, a decision analysis based on stochastic regression trees and Markov models compared cost-effectiveness of primary prophylaxis with beta-blockers (starting from the diagnosis of small varices) and endoscopic surveillance (and beta-blockers administration when large varices develop). Beta-blocker therapy from the beginning turned out to be more effective and less expensive. This result demonstrate that an early beta-blocker therapy besides being clinically effective, is also also cost-effective.
-
Citation: Di Pascoli L, Buja A, Bolognesi M, Montagnese S, Gatta A, Gregori D, Merkel C. Cost-effectiveness analysis of beta-blockers
vs endoscopic surveillance in patients with cirrhosis and small varices. World J Gastroenterol 2014; 20(30): 10464-10469 - URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10464.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10464
The natural history of portal hypertension in patients with liver cirrhosis is characterized by varices formation, progression of varices from small to large and, eventually, variceal rupture with upper gastrointestinal bleeding, which is associated with an increased risk of death[1-3]. Although there is general agreement that variceal hemorrhage is very unusual while varices remain small[4-6], it is reasonable to make an effort to avoid or delay the progression of small varices into large varices and, in turn, to prevent the occurrence of the first episode of variceal bleeding.
Pharmaco-economical studies suggest that treatment with beta-blockers is a reasonable strategy to prevent the first variceal bleed in cirrhosis, irrespective of disease severity[5,6], or in patients with advanced disease[7], even in the absence of endoscopic screening. However, this approach remains controversial and it has been questioned in a recent editorial[8] and in a survey amongst clinical experts during a consensus conference[9]. Thus, current guidelines still suggest endoscopic screening, to predict who will benefit from prophylactic treatment[10,11]. In patients with large esophageal varices, several clinical trials have shown that prophylaxis with beta-blockers is effective in reducing the risk of a first variceal bleed[12,13]. In patients with small varices, different studies have shown different results, but according a meta-analysis published in 2004, the use of beta-blockers seems to be useful in the prevention of first bleeding vs placebo[14]. So, most international guidelines “recommend” nonselective beta-blockers for the prevention of a first variceal bleed if the risk of hemorrhage is high (varices with red wale marks or Child C class), while they only “suggest” their use if the risk is not high[10,11]. Actually, there are no alternative procedures for the prevention of first varieceal bleeding in subjects with small varices and intolerance to beta-blockers. In recent years, the problem of defining patients suitable for treatment was also addressed using non-invasive scores predicting the presence of large varices[15,16].
In spite of the evidence gathered on the efficacy of treatment with beta-blokers, no study so far has evaluated the economical consequences of replacing traditional endoscopic surveillance with primary prophylaxis with beta-blockers in patients with compensated cirrhosis and small esophageal varices.
In this study, a decision analysis based on stochastic regression trees and Markov models has been conducted to compare the cost-effectiveness of primary prophylaxis with beta-blockers (starting from the diagnosis of small esophageal varices) and that of endoscopic surveillance. In this latter strategy, beta-blockers are administered when large varices develop.
Patients characteristics are given as frequencies, mean ± SD, or medians and interquartile ranges, where appropriate, and were compared using χ2, t test, or Mann Whitney Test, respectively. Significance level was set at P < 0.05.
A decision model based on stochastic regression trees was developed in order to evaluate the overall costs of early beta-blocker treatment vs endoscopic surveillance. The basic nodes of the tree were gastrointestinal endoscopy, inpatient admission and treatment for bleeding, as required.
All the relevant variables, such as the likelihood of bleeding and subsequent inpatient admission or mortality, along with their consequences in terms of costs, were integrated in the decision model. This allowed for the definition of expected benefits (i.e., model outcomes in terms of bleeding or progression episodes prevented), and additional costs incurred in or saved by early prescription of beta-blockers. The same model was reiterated using a Markov Process over a four-year follow-up period.
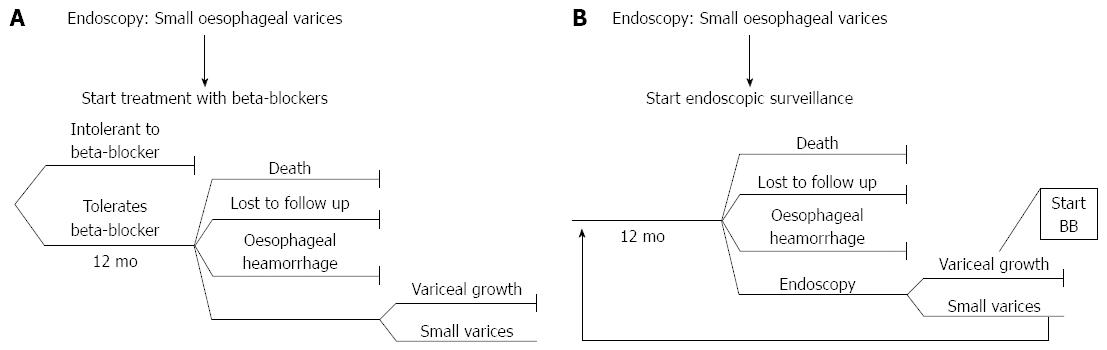
Within the decision model, the first branch of the tree represented the choice of whether to administer beta-blockers at an early stage (Strategy 1) (Figure 1A) or keep the patient under surveillance and no treatment until a follow-up endoscopy (planned on a 12-mo basis) was performed and treatment required (Strategy 2) (Figure 1B).
The analysis was performed by entering all relevant point estimates (i.e., the likelihood of bleeding, inpatient admission, death, costs, etc.) at each node, and by modeling them as expected values of binomial probabilities. Costs were modelled using a Gamma distribution. Thus the expected outcome distribution based on the two strategies was obtained. All estimates were performed using a Monte Carlo microsimulation technique, consisting in simulating observations from known probability distributions depicted in the model. Eight-hundred-thousand simulations were performed to obtain the final estimates.
All estimates were then subjected to Monte Carlo Probabilistic sensitivity analysis, to assess the impact of the variability of such estimates on the outcome distributions. The latter provided an indication of the robustness of the results obtained and was performed on 100000 samples from all distributions assumed in the tree. Comparative performances associated to Strategy 1 as compared to Strategy 2 were measured by the cost-effectiveness ratio. Discounting was not considered relevant because of the limited duration of the follow-up period. Cost-effectiveness analysis was performed as recommended by the Panel on Cost-Effectiveness in Health and Medicine[17,18].
All estimates are presented with 95% credibility intervals. The analyses were performed using TreeAge and the R-System[19].
The trial Placebo-controlled clinical trial of Nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis[3] (Table 1) and the hospital administrative database was utilised to derive the set of events and their probability. If unavailable, data were integrated based on other published studies. Table 2 shows the probabilistic scenarios adopted for purposes of model building and subsequent estimation.
| Strategy 1 patients | Strategy 2 patients | P value | ||
| (n = 83) | (n = 78) | |||
| Age (yr) | 56 ± 9 | 57 ± 9 | t = 1,26 | 0.21 |
| Sex (M/F) | 45/38 | 38/40 | χ2 = 0.49 | 0.48 |
| Aetiology (alcohol-related/viral/other) | 47/34/2 | 45/28/5 | χ2 = 0.02 | 0.89 |
| Hepatitis B surface antigen positive | 4 | 3 | corrχ2 = 0.01 | 0.93 |
| Time since diagnosis of cirrhosis (yr) | 3.1 ± 2.7 | 2.9 ± 2.8 | t = 0.282 | 0.78 |
| Time since diagnosis of varices (mo) | 2.9 ± 2.4 | 2.8 ± 2.5 | t = 0.022 | 0.98 |
| Child-Pugh score | 6 (IQR: 5-8) | 7 (IQR: 6-8) | Z = - 0.87 | 0.38 |
| Ascites | 18 | 23 | χ2 = 1.29 | 0.26 |
| Follow-up time (mo) | 36 ± 18 | 35 ± 15 | t = 0.325 | 0.75 |
| Variables | Baseline assumption | |||
| 12 mo | 24 mo | 36 mo | 48 mo | |
| Strategy 1 group | ||||
| Intolerance to nadolol | 4.8% | 2.9% | 0.0% | 2.5% |
| Death | 0.0% | 4.5% | 12.3% | 7.7% |
| Lost to follow-up | 10.1% | 7.5% | 12.3% | 23.1% |
| Esophageal hemorrhage | 0.0% | 0.0% | 0.0% | 0.0% |
| Variceal growth | 2.8% | 3.4% | 7.0% | 0.0% |
| Strategy 2 group: | ||||
| Death | 1.3% | 0.0% | 15.6% | 14.3% |
| Lost to follow-up | 6.4% | 6.7% | 11.1% | 21.2% |
| Esophageal hemorrhage | 1.3% | 1.7% | 0.0% | 0.0% |
| Variceal growth | 15.5% | 18.2% | 15.2% | 16.7% |
The event rate was computed with reference to the following, different sets of combined endpoints: (1) Number of bleeding episodes or deaths before the progression of varices; (2) Number of bleeding episodes or deaths before the progression of varices, plus number of progressions to large varices; and (3) Number of bleeding episodes or deaths before the progression of varices, plus number of progressions to large varices, plus number of bleeding episodes or deaths after the progression of varices.
Each intervention was associated to the United Kingdom NHS average costs. The cost of an upper gastrointestinal endoscopy is 164 £; the cost of a gastrointestinal bleeding episode is 3498 £. The monthly expenditure for nonselective beta-blockers tablets is 20.62 £ (2474 £/year). It was assumed that patients who stopped beta-blockers because of side effects had been on treatment for four months (8248 £).
The event rate considering bleeding and/or death prior to the progression of varices in the beta-blockers group was 15.66% (95%CI: 7.84%-23.48%), thus not significantly different from that in the surveillance group, which was 21.33 % (95%CI: 12.06%-30.60%). Therefore, the CER for this endpoint was 0.42 £ (95%CI: -3574.09 £-3573.25 £) per event avoided; The event rate for this second, combined endpoint was significantly lower in the beta-blockers group [24.09% (95%CI: 14.89%-33.29%)], compared to the surveillance group [60.00% (95%CI: 48.91%-71.08%)]. The mean cost (up to the first event) associated with early beta-blockers treatment was 823 £ (95%CI: 106 £-2036 £), which was not significantly different from that of surveillance [799 £ (95%CI: 0 £-3498 £)]. Therefore, the cost-effectiveness ratio (CER) for this endpoint was 50.26 £ (95%CI: -504.37 £-604.89 £) per event avoided over the four-year follow-up; and the event rate for this third, combined endpoint was significantly lower in the beta-blockers group [29.55% (95%CI: 18.88%-38.27%)], compared to the surveillance group [62.40% (95%CI: 50.93%-71.87%)].
If a bleeding episode and death occurring in subjects with variceal growth were included in the analysis of costs, then the mean cost associated with early treatment was 1028 £ (95%CI: 122 £-2581 £), and that associated with traditional surveillance 1699 £ (95%CI: 171 £-4674 £).
Sensitivity analysis was consistent with the results above. For all cost analyses, the mean values never differed from the above estimates of more than 17% of the highest cost. Confidence intervals were also consistently providing the same indications as above.
Cost-effectiveness evaluations of different strategies for the primary prophylaxis of variceal bleeding have already been performed in patients with large varices[20], and in patients with cirrhosis, irrespective of the presence of varices[5-7]. No study to date has evaluated the cost-effectiveness of prophylaxis of small esophageal varices, comparing early beta-blocker treatment institution with endoscopic surveillance plus beta-blocker treatment when varices increase in size.
Cost-effectiveness analysis may result in different estimates, depending on the set of events considered. For this reason, in this study combined endpoints related to different, potential actions of beta-blockers were analysed. Firstly, the number of bleeding episodes and/or deaths prior to the diagnosis of variceal progression were considered. Within this setting, and in agreement with previous findings[3], early beta-blockers were found not to be effective in preventing bleeding episodes/deaths before the diagnosis of variceal enlargement. This is probably related to: (1) the fact that variceal hemorrhage is rare when varices are small[4]; and (2) beta-blockers do not affect overall survival because bleeding is not the main cause of death in a patient with cirrhosis. Despite this lack of effectiveness, the present analysis demonstrates that the institution of early treatment with beta-blockers is not more expensive than endoscopic surveillance.
If we consider that variceal hemorrhage occurs at a yearly rate of 5%-15%, and its main predictor is variceal size, with the highest risk of first hemorrhage (15% per year) in patients with large varices[21,22], it is reasonable to assume that preventing variceal enlargement is equivalent to preventing its consequences, i.e., bleeding and bleeding-related death. Thus, in our second analysis, the progression of varices was added to the “events” bleeding and death prior to variceal growth. Based on this analysis, beta-blockers were more effective, and their cost was about one pound per month per event avoided, which seems very reasonable.
Since the most important clinical endpoint underlying the selection of the best strategy for these patients is the prevention of overall bleeding episodes/deaths, prior to and after variceal growth, all such events were included in the third analysis. Within this setting, the number of events associated to the early treatment strategy was obviously smaller compared to that of the surveillance strategy. In addition, the relative costs of early treatment were lower as this strategy was associated with a lower incidence of bleeds after progression, due to the fewer progressions. Thus, overall, the early institution of treatment with beta-blockers turns out to be not only more effective, but also less expensive. This suggests that treatment with beta-blockers impinges on both the natural history of a patient with cirrhosis and small varices and also on health costs.
Nonselective betablockers are recommended in patients with small varices only if there are red wale marks or the patients are classed as Child C, while they are only “suggested” if no such risk factors are present[10,11]. Considering the good safety profile of beta-blockers and the costs and unpleasantness of endoscopic surveillance, these findings support the use of beta-blockers as the preferred therapy for the prophylaxis of the first variceal bleeding in all patients with cirrhosis and small esophageal varices. Since endoscopic variceal ligation has never been suggested for primary prophylaxis of variceal bleeding in patients with small varices, it would appear that early prophylaxis with beta-blocker is, at present, the only feasible strategy within this clinical scenario.
Cost-effectiveness analysis may provide different results depending on different payment models and different costs for the procedures. In the present analysis, United Kingdom, crude average costs were utilised, as they are based on allowable reimbursement costs and direct, published cost estimates. However, in the sensitivity analyses the CER therapy remained cost-effective within the tested range. These data suggest that beta-blockers may be cost-effective also in different payment models, for instance the Italian one.
In conclusion, early institution of treatment with beta-blockers, being more effective and less expensive, appears to be “dominant” over endoscopic surveillance for the primary prophylaxis of bleeding in patients with cirrhosis and small esophageal varices who tolerate this treatment.
Variceal hemorrhage is unusual when varices are small. Thus, it is reasonable to make every effort to delay their progression. In patients with cirrhosis and small varices, international guidelines “recommend” beta-blockers for the prevention of first bleed if the risk of hemorrhage is high (i.e., when varices are large), while they “suggest” their use if risk is low (i.e., when varices are small).
In consideration of the frequent occurrence of small varices in overall clinical practice, beta-blocker treatment should be also evaluated from a cost- effectiveness point of view. No economical evaluation for this strategy has been performed to date.
In this study, a decision analysis based on stochastic regression trees and Markov models compared cost-effectiveness of primary prophylaxis with beta-blockers (starting from the diagnosis of small varices) and endoscopic surveillance (and beta-blockers administration when large varices develop). Beta-blocker therapy from the beginning turned out to be more effective and less expensive.
This result demonstrate that an early beta-blocker therapy besides being clinically effective, is also also cost-effective. This implies that the treatment of patients with esophageal varices starting from the condition of small varices may be suggested for this clinical situation.
Cost-effectiveness: a form of economic analysis that compares the relative costs and outcomes (effects) of two or more courses of action.
In this paper, the authors analyse the cost-effectiveness of beta-blockers in surveillance and outcome of early esophageal varices in patients with liver cirrhosis compared to endoscopy measures. The authors provide a clear rational for the study set-up and give detailed statistical descriptions of the methods. The manuscript is well written and the results are presented concise but clear. The discussion is to the point and highlights the current knowledge.
P- Reviewer: Borgia G, Ocker M S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Pagliaro L, D’Amico G, Pasta L, Tiné F, Aragona E, Politi F, Malizia G, Puleo A, Peri V, D’Antoni A. Efficacy and efficiency of treatments in portal hypertension. Portal hypertension II. London, England: Black-well 1996; 159-179. [Cited in This Article: ] |
| 2. | Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, Attili AF, Riggio O. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 3. | Merkel C, Marin R, Angeli P, Zanella P, Felder M, Bernardinello E, Cavallarin G, Bolognesi M, Donada C, Bellini B. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology. 2004;127:476-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Zoli M, Merkel C, Magalotti D, Gueli C, Grimaldi M, Gatta A, Bernardi M. Natural history of cirrhotic patients with small esophageal varices: a prospective study. Am J Gastroenterol. 2000;95:503-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Saab S, DeRosa V, Nieto J, Durazo F, Han S, Roth B. Costs and clinical outcomes of primary prophylaxis of variceal bleeding in patients with hepatic cirrhosis: a decision analytic model. Am J Gastroenterol. 2003;98:763-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Spiegel BM, Targownik L, Dulai GS, Karsan HA, Gralnek IM. Endoscopic screening for esophageal varices in cirrhosis: Is it ever cost effective? Hepatology. 2003;37:366-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Arguedas MR, Heudebert GR, Eloubeidi MA, Abrams GA, Fallon MB. Cost-effectiveness of screening, surveillance, and primary prophylaxis strategies for esophageal varices. Am J Gastroenterol. 2002;97:2441-2452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Rubenstein JH, Inadomi JM. Empiric beta-blockers for the prophylaxis of variceal hemorrhage: cost effective or clinically applicable? Hepatology. 2003;37:249-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Groszmann RJ, Merkel C, Iwakiri Y, Shah V, Shneider BJ, Zoli M, Berzigotti A, Vorobioff J, Morabito A. Prevention of the formation of varices (pre-primary prophylaxis). Portal Hypertension IV. Oxford: Blackwell 2006; 103-151. [Cited in This Article: ] |
| 10. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1229] [Cited by in F6Publishing: 1147] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 11. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 977] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 12. | D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 592] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 13. | Conn HO, Grace ND, Bosch J, Groszmann RJ, Rodés J, Wright SC, Matloff DS, Garcia-Tsao G, Fisher RL, Navasa M. Propranolol in the prevention of the first hemorrhage from esophagogastric varices: A multicenter, randomized clinical trial. The Boston-New Haven-Barcelona Portal Hypertension Study Group. Hepatology. 1991;13:902-912. [PubMed] [Cited in This Article: ] |
| 14. | Merkel C, Bolognesi M, Gatta A. The cirrhotic patient with no varices and with small varices. Portal Hypertension in the 21st Century. Dordrecht: Kluver Academic Publishers 2004; 271-276. [Cited in This Article: ] |
| 15. | Adami MR, Ferreira CT, Kieling CO, Hirakata V, Vieira SM. Noninvasive methods for prediction of esophageal varices in pediatric patients with portal hypertension. World J Gastroenterol. 2013;19:2053-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Berzigotti A, Gilabert R, Abraldes JG, Nicolau C, Bru C, Bosch J, García-Pagan JC. Noninvasive prediction of clinically significant portal hypertension and esophageal varices in patients with compensated liver cirrhosis. Am J Gastroenterol. 2008;103:1159-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1584] [Cited by in F6Publishing: 1490] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 18. | Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 789] [Cited by in F6Publishing: 740] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 19. | Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing 2010; . [Cited in This Article: ] |
| 20. | Imperiale TF, Klein RW, Chalasani N. Cost-effectiveness analysis of variceal ligation vs. beta-blockers for primary prevention of variceal bleeding. Hepatology. 2007;45:870-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 927] [Cited by in F6Publishing: 793] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 22. | Merkel C, Zoli M, Siringo S, van Buuren H, Magalotti D, Angeli P, Sacerdoti D, Bolondi L, Gatta A. Prognostic indicators of risk for first variceal bleeding in cirrhosis: a multicenter study in 711 patients to validate and improve the North Italian Endoscopic Club (NIEC) index. Am J Gastroenterol. 2000;95:2915-2920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |