Published online Nov 14, 2012. doi: 10.3748/wjg.v18.i42.6141
Revised: September 18, 2012
Accepted: September 29, 2012
Published online: November 14, 2012
AIM: To investigate the liver-protecting effect of parenteral nutrition (PN) support with omega-3 fatty acids in a randomized controlled clinical trial.
METHODS: Sixty-six patients with the diagnosis of end-stage liver disease or hepatic cellular carcinoma were admitted to the Affiliated Drum Tower Hospital, Nanjing University, China for orthotopic liver transplantation. The patients were randomly divided into two groups: PN group (n = 33) and polyunsaturated fatty acid (PUFA) group (n = 33). All patients received isocaloric and isonitrogenous PN for seven days after surgery, and in PUFA group omega-3 fish oil lipid emulsion replaced part of the standard lipid emulsion. Liver function was tested on days 2 and 9 after surgery. Pathological examination was performed after reperfusion of the donor liver and on day 9. Clinical outcome was assessed based on the post-transplant investigations, including: (1) post-transplant mechanical ventilation; (2) total hospital stay; (3) infectious morbidities; (4) acute and chronic rejection; and (5) mortality (intensive care unit mortality, hospital mortality, 28-d mortality, and survival at a one-year post-transplant surveillance period).
RESULTS: On days 2 and 9 after operation, a significant decrease of alanine aminotransferase (299.16 U/L ± 189.17 U/L vs 246.16 U/L ± 175.21 U/L, P = 0.024) and prothrombin time (5.64 s ± 2.06 s vs 2.54 s ± 1.15 s, P = 0.035) was seen in PUFA group compared with PN group. The pathological results showed that omega-3 fatty acid supplement improved the injury of hepatic cells. Compared with PN group, there was a significant decrease of post-transplant hospital stay in PUFA group (18.7 d ± 4.0 d vs 20.6 d ± 4.6 d, P = 0.041). Complications of infection occurred in 6 cases of PN group (2 cases of pneumonia, 3 cases of intra-abdominal abscess and 1 case of urinary tract infection), and in 3 cases of PUFA group (2 cases of pneumonia and 1 case of intra-abdominal abscess). No acute or chronic rejection and hospital mortality were found in both groups. The one-year mortality in PN group was 9.1% (3/33), one died of pulmonary infection, one died of severe intra-hepatic cholangitis and hepatic dysfunction and the other died of hepatic cell carcinoma recurrence. Only one patient in PUFA group (1/33, 3.1%) died of biliary complication and hepatic dysfunction during follow-up.
CONCLUSION: Post-transplant parenteral nutritional support combined with omega-3 fatty acids can significantly improve the liver injury, reduce the infectious morbidities, and shorten the post-transplant hospital stay.
- Citation: Zhu XH, Wu YF, Qiu YD, Jiang CP, Ding YT. Liver-protecting effects of omega-3 fish oil lipid emulsion in liver transplantation. World J Gastroenterol 2012; 18(42): 6141-6147
- URL: https://www.wjgnet.com/1007-9327/full/v18/i42/6141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i42.6141
Liver transplantation has dramatically improved the prognosis of end-stage liver disease. The progress made in the immunosuppressive regimens and surgical techniques has yielded a better outcome of the patients, and the 5-year survival after liver transplantation is 70%-80%[1]. The liver recipients with liver insufficiency are in fact known to experience a higher incidence of severe protein/calorie malnutrition, which is associated with a greater risk of postoperative complications and mortality in patients undergoing liver transplantation[2,3]. And ischemia/reperfusion (I/R) injury associated with liver transplantation often leads to hepatic dysfunction despite the improvement in surgical techniques and perioperative medication. I/R injury of the liver is an event involving multiple factors, such as hypoxia during inflow occlusion of the liver and inflammatory reactions after reperfusion[4,5], and the mechanisms of the reperfusion injury, including the release of inflammatory cytokines, the generation of oxygen free radicals, Kupffer cell activation and leukocyte-endothelial cell interaction[6,7]. Based on the pathophysiology of hepatic I/R injury, the current study particularly focused on various perioperative approaches to protect the liver from these inflammatory reactions and microcirculatory disturbances.
Omega-3 (N-3) fatty acids, which are derived from fish oil, are essential polyunsaturated fatty acids (PUFAs) for humans. Omega-3 fatty acids exert anti-inflammatory and immunomodulatory properties through their ability to modulate the synthesis of different eicosanoids[8,9]. Perioperative administration of omega-3 fatty acids reduces plasma and tissue levels of the eicosanoids, specific leukotrienes, thromboxanes, and prostaglandins, all of which have pro-inflammatory effects[10,11]. Recent studies described that supplementation with omega-3 fatty acids decreases the rate of inflammatory complications, the length of hospital stay, and the mortality after major abdominal surgeries[12-14]. Their protective effects on hepatic I/R injury and inflammatory responses have been increasingly investigated.
In this study, we investigated the liver-protecting effects of parenteral nutrition (PN) supplemented with omega-3 fish oil lipid emulsion in patients undergoing liver transplantation.
This study was carried out in the Department of Hepatobiliary Surgery of the Affiliated Drum Tower Hospital, Medical School of Nanjing University, China according to the principles and guidelines of the Helsinki Declaration of 1975 revised in 2000. The protocol was approved by the Ethics Committee of the Affiliated Drum Tower Hospital. All patients fully understood the objective and adverse reactions of the study, and signed the written informed consent voluntarily prior to study enrollment.
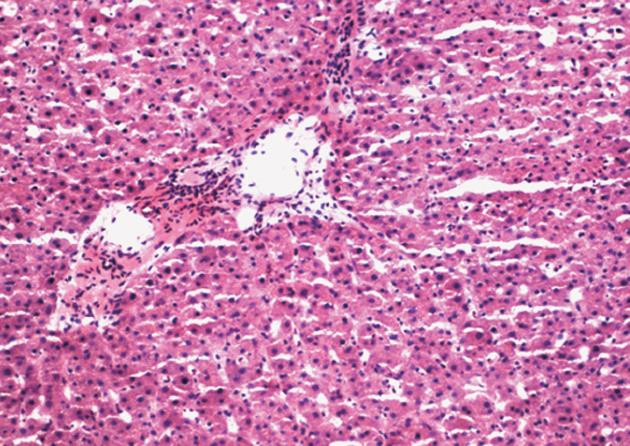
From January 2006 to July 2010, we prospectively investigated 66 patients (45 men and 21 women; mean age, 51.6 years; range, 34-64 years) who underwent orthotopic liver transplantation in the Department of Hepatobiliary Surgery at the Affiliated Drum Tower Hospital of Medical School of Nanjing University. Recipients with manifest metabolic diseases (e.g., diabetes mellitus and hyperthyroidism) or severe renal abnormality were excluded. No acute rejection, primary transplanted liver dysfunction or second operation, which may affect the evaluation of liver function, were seen during the first 9 d after transplantation. The selection criteria for donors in this study included: (1) age < 50 years, matched ABO blood group and no history of chronic liver disease; (2) no evidence of malignant tumor, viral hepatitis or other viral infections; (3) no cirrhosis, mass or severe fatty degeneration of the donor liver seen during organ harvesting; and (4) liver biopsies of each donor liver taken before transplantation were reviewed by two pathologists. Donor livers with normal pathology or mild fatty change (10%-30%) were included in this study (Figure 1).
After transplantation, these patients were randomized into two groups based on the randomization chart generated by the Statistical Analysis System (SAS): (1) PN group (33 patients), without supplementation of omega-3 fatty acids in addition to routine treatment; and (2) PUFA group (33 patients), with PN supplemented with omega-3 fatty acids in addition to routine treatment.
This is a randomized controlled clinical study carried out in the Department of Hepatobiliary Surgery of our hospital. Nutrition Risk Screening 2002 (NRS 2002) scoring system was used, and the post-operative NRS 2002 score was ≥ 3 in all the patients, which meant that all the patients need nutritional support.
The PN was given around the clock for seven days from the second day after operation. The two nutritional support groups were isonitrogenous and isocaloric. Nitrogen intake was 0.16 g/kg body weight per day, caloric intake was 104.5 kJ/kg per day, and lipid intake was 1.0 g/kg per day. The nonprotein calories were provided with dextrose (4.0 g/kg per day) and fat emulsion in a ratio of 2:1. The only source of lipids in PN group was the standard lipid emulsion (20% emulsion, with a ratio of long-chain triglycerides to medium-chain triglycerides of 1:1, Huarui Pharmaceuticals, Jiangsu, China), and in PUFA group omega-3 fish oil lipid emulsion (Omegaven, 10%, 2 mL/kg per day, Fresenius Kabi Co., Austria) replaced part of the standard lipid emulsion. Both groups received 1.0 g amino acid/kg per day, and they were administered a commercially available branched-chain amino acid solution (Branched-chain amino acid solution 20%, Huarui Pharmaceuticals, Jiangsu, China). The ratio of nonprotein calories to nitrogen in both nutritional support groups was 653 kJ:1 g. The omega-3 fish oil lipid emulsion-containing solutions were prepared by the clinical pharmacist under aseptic condition and adjusted according to the weight of each individual patient. The amino acids, fat emulsion and dextrose mixture with electrolytes, vitamins, and trace elements were administered through a central venous catheter. As soon as the bowel function returned on days 3 or 4 after transplantation, all patients in the two groups were given liquid carbohydrate and cow’s milk protein.
The surgical treatment was standardized, and modified piggyback orthotopic liver transplantation was performed by three groups of surgeons using the same approach. After operation, all the patients in the two groups were treated with the same antibiotics and antivirutics, and 20 g of albumin was administered intravenously daily for five days to prevent any complications caused by hypoalbuminemia.
Venous heparin blood samples were obtained on days 1 (the day before transplantation), 2 and 9 after surgery and liver function assessment was made. Serum total bilirubin (TB), direct bilirubin (DB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and prothrombin time (PT) were measured by an automatic biochemical analyzer (HITACHI 7600, Japan).
Liver biopsy with fine needle was conducted after reperfusion of donor liver and on day 9 after surgery, respectively. Hepatic specimens for light microscopy were fixed with formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin for histological examination. Portal inflammation in the liver biopsy specimens was semiquantified by calculating inflammatory cells in portal tracts based on the Knodell histology activity index (HAI)[15]. Portal inflammation was scored as 0, no portal inflammation; 1, mild (sprinkling of inflammatory cells in < 1/3 of portal tracts); 3, moderate (increased inflammatory cells in 1/3-2/3 of portal tracts); and 4, marked (dense packing of inflammatory cells in > 2/3 of portal tracts).
The assessment of clinical outcome was based on post-transplant investigations as shown by: (1) post-transplant mechanical ventilation; (2) total hospital stay; (3) infectious morbidities (pneumonia, intra-abdominal abscess, central line sepsis, wound infection, and urinary tract infection); (4) acute and chronic rejection; (5) mortality (intensive care unit mortality, hospital mortality, 28-d mortality, and survival at one year post-transplant surveillance period).
These post-transplant parameters were investigated and documented daily during the patients’ post-transplant hospital stay and the period of one-year postoperative follow-up.
The results were expressed as mean ± SD. Data were analyzed using the SAS. Differences between means were evaluated using Student t test when normal distribution was confirmed by Shapiro-Wilks test. When the hypothesis of normal distribution was rejected, differences between groups were tested by nonparametric statistics using Mann-Whitney test for unpaired samples and Wilcoxon criteria for paired samples. Fisher’s exact test was used for analysis of categorical values when appropriate. A P value of < 0.05 was considered significant.
A total of 66 patients were enrolled in this study, including 33 patients in PN group and 33 patients in PUFA group. The mean age of the subjects was 51.6 years (range, 34-64 years). The clinical diagnosis of these patients included: hepatic cell carcinoma (27 cases), post-hepatitis B liver cirrhosis (35 cases), alcoholic liver cirrhosis (1 case), primary biliary liver cirrhosis (2 cases) and congenital polycystic liver (1 case). Demographic and clinical data (including age, sex, clinical diagnosis, Child-Pugh classification of hepatic function, warm ischemic time, cold ischemic time, operative time, anhepatic phase and post-operative immunosuppression) are summarized in Table 1. With respect to warm ischemic time, cold ischemic time, operative time, anhepatic phase, ratio of Child-Pugh classification, immunosuppression and clinical diagnosis, there were no significant differences between the two groups in any of these above parameters (P > 0.05).
| PN group | PUFA group | |
| Sex (M/F) | 23/10 | 22/11 |
| Age, yr | 48.62 ± 14.61 | 51.52 ± 12.41 |
| Clinical diagnosis | ||
| Hepatic cell carcinoma | 13 | 14 |
| Post- hepatitis B liver cirrhosis | 17 | 18 |
| Alcoholic liver cirrhosis | 1 | 0 |
| Primary biliary liver cirrhosis | 1 | 1 |
| Congenital polycystic liver | 1 | 0 |
| Child-Pugh classification (A/B/C) | 14/10/9 | 13/10/10 |
| Warm ischemic time (min) | 3.91 ± 1.16 | 4.15 ± 1.32 |
| Cold ischemic time (min) | 524.28 ± 132.83 | 506.56 ± 151.26 |
| Operation period (min) | 651.27 ± 181.42 | 626.39 ± 192.86 |
| Anhepatic phase (min) | 119.81 ± 82.35 | 142.15 ± 58.75 |
| Immunosuppressive therapy | 22/11/0 | 21/11/1 |
| (FK506 + P/CSA + P/CSA + P + MMF) |
No significant difference of pre-operative liver function was observed between the two groups. On days 2 and 9 after operation, a significant decrease of ALT (299.16 U/L ± 189.17 U/L vs 246.16 U/L ± 175.21 U/L, P = 0.024) and PT (5.64 s ± 2.06 s vs 2.54 s ± 1.15 s, P = 0.035) was seen in PUFA group compared with PN group. And there was no significant decrease of the following parameters tested on days 2 and 9: AST (116.31 U/L ± 42.19 U/L vs 121.09 U/L ± 53.14 U/L, P=0.682), TB (93.93 μmol/L ± 45.49 μmol/L vs 87.20 μmol/L ± 61.12 μmol/L, P = 1.439), DB (42.74 μmol/L ± 17.36 μmol/L vs 36.22 μmol/L ± 21.63 μmol/L, P = 0.815) and LDH (156.12 U/L ± 89.20 U/L vs 119.10 U/L ± 69.72 U/L, P = 1.112) in PUFA group compared with PN group (Table 2).
| Normal value | Group | Day 1 | Day 2 | Day 9 | Decrease (Day 2-Day 9) | |
| ALT (U/L) | 5-40 | PN group | 198.16 ± 117.13 | 401.32 ± 215.35 | 155.16 ± 108.41b | 246.16 ± 175.21 |
| PUFA group | 227.16 ± 121.17 | 410.98 ± 201.64 | 101.82 ± 71.24b | 299.16 ± 189.17c | ||
| AST (U/L) | 8-40 | PN group | 95.12 ± 61.79 | 203.25 ± 73.49 | 82.16 ± 46.16b | 121.09 ± 53.14 |
| PUFA group | 115.62 ± 81.27 | 185.12 ± 42.16 | 68.81 ± 24.32b | 116.31 ± 42.19 | ||
| TB (μmol/L) | 5-20.5 | PN group | 92.16 ± 42.15 | 158.32 ± 65.41 | 71.12 ± 55.12a | 87.20 ± 61.12 |
| PUFA group | 116.82 ± 61.65 | 160.34 ± 68.24 | 66.41 ± 61.52b | 93.93 ± 45.49 | ||
| DB (μmol/L) | 1.7-6.8 | PN group | 52.15 ± 32.95 | 76.46 ± 31.28 | 40.24 ± 26.69b | 36.22 ± 21.63 |
| PUFA group | 47.39 ± 27.19 | 81.25 ± 26.32 | 38.51 ± 19.87b | 42.74 ± 17.36 | ||
| LDH (U/L) | 109-245 | PN group | 266.25 ± 132.42 | 476.25 ± 98.15 | 357.15 ± 192.52a | 119.10 ± 69.72 |
| PUFA group | 226.45 ± 172.24 | 416.38 ± 151.14 | 260.26 ± 111.32b | 156.12 ± 89.20 | ||
| PT (s) | 10-15 | PN group | 18.76 ± 3.21 | 17.16 ± 4.05 | 14.62 ± 3.87a | 2.54 ± 1.15 |
| PUFA group | 19.12 ± 4.16 | 17.81 ± 3.82 | 12.17 ± 3.69b | 5.64 ± 2.06c |
The histological examination after reperfusion revealed some swelling hepatocytes and inflammatory cell infiltration in the portal areas, and no significant difference of numerical score of portal inflammation was observed between the two groups.
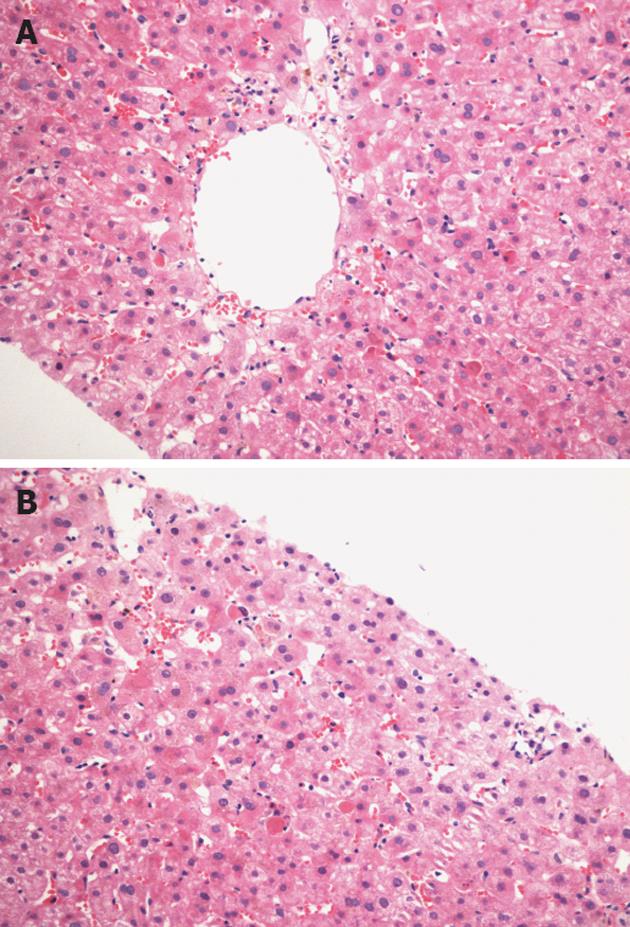
Histological examination on day 9 in PN group revealed more inflammatory cells aggregating in hepatic sinusoid lumen, extensive swelling and some balloon-like degeneration of hepatocytes, extensive congestion, and bilirubin deposit in the hepatic plasma (Figure 2A). These were ameliorated markedly by parenteral nutritional support with omega-3 fatty acids (Figure 2B), and the numerical score of portal inflammation was significantly lowered in PUFA group (Table 3). There was no sign of acute rejection in both groups.
There was no significant difference of post-transplant mechanical ventilation between the two groups (P > 0.05). Compared with PN group, the post-transplant hospital stay was significantly shortened in PUFA group (P < 0.05). Infectious complications occurred in 6 cases of PN group (2 cases of pneumonia, 3 cases of intra-abdominal abscess and 1 case of urinary tract infection), and in 3 cases of PUFA group (2 cases of pneumonia, 1 case of intra-abdominal abscess). No acute or chronic rejection and hospital mortality were found in the two groups. All patients were followed up, and the one-year mortality in PN group was 9.1% (3/33), one died of pulmonary infection, one died of severe intra-hepatic cholangitis and hepatic dysfunction and the other of hepatic cell carcinoma recurrence. Only one patient in PUFA group (1/33, 3.1%) died of biliary complication and hepatic dysfunction during follow-up (Table 4).
An impairment of nutritional status is a frequent finding in patients with end-stage liver disease. Malnutrition adversely affects the prognosis of these patients and is associated with the morbidity and mortality after liver transplantation[16]. Malnutrition has been shown to be the only independent risk factor for the length of stay in the intensive care and the total number of days spent in the hospital, and the liver recipient’s nutritional status also influences the incidence of post-transplant complications and may therefore increase the costs of liver transplant[17,18]. After liver transplantation, surgical stress, postoperative fasting, and the possible occurrence of complications suggest the need for nutritional support. The primary goal of the nutrition support in the immediate post-transplant period is to provide adequate nutrition to promote recovery and replenishment of the depleted nutrient stores. Although most transplant centers use the similar post-transplant nutritional support as for other major abdominal operations, few studies have elucidated the role of postoperative nutritional support in the liver recipients.
Enteral nutrition is safer and less expensive than PN, and enteral nutrition has the potential advantage of maintaining intestinal trophism more effectively[19]. This effect may help prevent bacterial translocation and enteric-origin infections in patients treated with transplantation[20,21]. All patients in this study resumed their daily oral diet postoperatively as soon as bowel function returned to maintain intestinal trophism, but the recipients could not endure a large amount of liquid diet even with nasoenteric tube at the early phase after transplantation because of obvious abdominal pain, distention or diarrhea in our previous experience. The bowel function in all the patients in this study returned on day 3 or 4 after transplantation, and PN support discontinued on day 8 after surgery when the patients were able to maintain an adequate oral intake.
Omega-3 fatty acids mainly act as eicosapentaenoic acids (EPA) and docosahexaenoic acids (DHA), both had anti-inflammatory effects. EPA and DHA reduce the release of arachidonic acid-derived pro-inflammatory eicosanoids, and generate a group of lipid mediators called resolvins (E- and D-series) and protectins with potent anti-inflammatory and inflammation resolution properties[22,23]. Studies with experimental models of liver reperfusion injury have reported the beneficial actions of n-3 PUFA-derived resolvins and protectins in preventing liver DNA damage and oxidative stress, thus significantly ameliorating the necroinflammatory liver injury and hepatic steatosis[24-26]. The liver-protecting effects of postoperative PN support supplemented with omega-3 fatty acids were evaluated in this study. Liver enzyme of ALT released after I/R was significantly suppressed by the supplements of omega-3 fatty acids. PT, as an important parameter in evaluating the synthesis function of liver, was significantly decreased in PUFA group. And the results of histological examination on day 9 revealed that the hepatocyte injury and inflammatory cell aggregation were ameliorated markedly in PUFA group. PUFA therapy could also decrease the infectious morbidities, and shorten the post-transplant hospital stay significantly. The possible mechanisms of omega-3 fatty acids include down-regulation of the inflammatory responses to surgery and immune modulation rather than a sole nutritional effect.
Some of the patients exhibited an obvious nitrogen accumulation disorder reflected by either encephalopathy or an excessive rise in blood urea nitrogen in the immediate postoperative period. Branched-chain amino acids were chosen for this study because it can promote protein synthesis in patients with chronic liver diseases and avoid the additional metabolic load of transplanted liver[27]. Medium-chain triglycerides were included in the regimen to avert glucose intolerance and deposits in the transplanted liver. Based on the results of this study, we think that post-transplant nutritional support in the form of a solution enriched with branched-chain amino acids, dextrose, medium-chain triglycerides and omega-3 fatty acids might offer a benefit in terms of preserved liver function and better clinical outcome, including the decreased infectious morbidities and post-transplant hospital stay.
In conclusion, we have shown that omega-3 fatty acids-supplemented PN significantly improves the injury of transplanted liver, decreases the infectious morbidities, and shortens the post-transplant hospital stay.
The authors wish to thank Dr. Chen Jun for his contribution to the pathological analysis.
The liver recipients with liver insufficiency are known to experience a higher incidence of severe protein/calorie malnutrition, and malnutrition is associated with a greater risk of postoperative complications and mortality in patients undergoing liver transplantation. And ischemia/reperfusion injury associated with liver transplantation often leads to hepatic dysfunction despite the improvements in surgical techniques and perioperative medication.
Omega-3 fatty acids exert anti-inflammatory and immunomodulatory properties through their ability to modulate the synthesis of different eicosanoids. Recent studies have described that supplementation with omega-3 fatty acids decreases the rate of inflammatory complication, the length of hospital stay, and the mortality after major abdominal surgeries.
Although most transplant centers use the similar post-transplant nutritional support as for other major abdominal operations, few studies have elucidated the role of postoperative nutritional support in the organ recipient. Based on the results of this study, the authors have shown that post-transplant nutritional support in the form of a solution enriched with branched-chain amino acids, dextrose, medium-chain triglycerides and omega-3 fatty acids might offer a benefit in terms of preserved liver function and better clinical outcome, including the decreased infectious morbidities and post-transplant hospital stay.
This study has shown that omega-3 fatty acids-supplemented parenteral nutrition (PN) significantly improves the injury of transplanted liver, decreases the infectious morbidities, and shortens the post-transplant hospital stay. The nutritional support strategy is recommended in patients undergoing liver transplantation.
The manuscript evaluates the potential for supplementation with polyunsaturated fatty acid to ameliorate hepatic injury associated with reperfusion and PN. It provides evidences about an efficient nutritional support strategy for liver transplanted patients.
Peer reviewer: Eric Kallwitz, Assistant Professor, Loyola University Medical Center, Chicago, IL 60612, United States
S- Editor Lv S L- Editor A E- Editor Li JY
| 1. | Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29:3-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Merli M, Nicolini G, Angeloni S, Riggio O. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002;18:978-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Millwala F, Nguyen GC, Thuluvath PJ. Outcomes of patients with cirrhosis undergoing non-hepatic surgery: risk assessment and management. World J Gastroenterol. 2007;13:4056-4063. [PubMed] [Cited in This Article: ] |
| 4. | Lee LY, Kaizu T, Toyokawa H, Zhang M, Ross M, Stolz DB, Huang C, Gandhi C, Geller DA, Murase N. Carbon monoxide induces hypothermia tolerance in Kupffer cells and attenuates liver ischemia/reperfusion injury in rats. Liver Transpl. 2011;17:1457-1466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Shen XD, Ke B, Uchida Y, Ji H, Gao F, Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Native macrophages genetically modified to express heme oxygenase 1 protect rat liver transplants from ischemia/reperfusion injury. Liver Transpl. 2011;17:201-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Schmidt R. Hepatic organ protection: from basic science to clinical practice. World J Gastroenterol. 2010;16:6044-6045. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Briceño J, Ciria R. Early graft dysfunction after liver transplantation. Transplant Proc. 2010;42:631-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res. 2012;51:232-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Han YY, Lai SL, Ko WJ, Chou CH, Lai HS. Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. Nutr Clin Pract. 2012;27:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280-289. [PubMed] [Cited in This Article: ] |
| 11. | Seki H, Tani Y, Arita M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 2009;89:126-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Nakamura K, Kariyazono H, Komokata T, Hamada N, Sakata R, Yamada K. Influence of preoperative administration of omega-3 fatty acid-enriched supplement on inflammatory and immune responses in patients undergoing major surgery for cancer. Nutrition. 2005;21:639-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Xu J, Yunshi Z, Li R. Immunonutrition in surgical patients. Curr Drug Targets. 2009;10:771-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Figueiredo F, Dickson ER, Pasha T, Kasparova P, Therneau T, Malinchoc M, DiCecco S, Francisco-Ziller N, Charlton M. Impact of nutritional status on outcomes after liver transplantation. Transplantation. 2000;70:1347-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31:241-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 340] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, Corradini SG, Siciliano M, Farcomeni A, Attili AF. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | Chen Y, Liu BL, Shang B, Chen AS, Liu SQ, Sun W, Yin HZ, Yin JQ, Su Q. Nutrition support in surgical patients with colorectal cancer. World J Gastroenterol. 2011;17:1779-1786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Thuluvath PJ. Morbid obesity and gross malnutrition are both poor predictors of outcomes after liver transplantation: what can we do about it? Liver Transpl. 2009;15:838-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Yuan Y, Ren J, Gu G, Chen J, Li J. Early enteral nutrition improves outcomes of open abdomen in gastrointestinal fistula patients complicated with severe sepsis. Nutr Clin Pract. 2011;26:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Yang H, Feng Y, Sun X, Teitelbaum DH. Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann N Y Acad Sci. 2009;1165:338-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Azuma H, Mishima S, Oda J, Homma H, Sasaki H, Hisamura M, Ohta S, Yukioka T. Enteral supplementation enriched with glutamine, fiber, and oligosaccharide prevents gut translocation in a bacterial overgrowth model. J Trauma. 2009;66:110-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S-1519S. [PubMed] [Cited in This Article: ] |
| 23. | Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 24. | Marsman HA, Heger M, Kloek JJ, Nienhuis SL, ten Kate FJ, van Gulik TM. Omega-3 fatty acids reduce hepatic steatosis and consequently attenuate ischemia-reperfusion injury following partial hepatectomy in rats. Dig Liver Dis. 2011;43:984-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Iwasaki W, Kume M, Kudo K, Uchinami H, Kikuchi I, Nakagawa Y, Yoshioka M, Yamamoto Y. Changes in the fatty acid composition of the liver with the administration of N-3 polyunsaturated fatty acids and the effects on warm ischemia/reperfusion injury in the rat liver. Shock. 2010;33:306-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Zúñiga J, Venegas F, Villarreal M, Núñez D, Chandía M, Valenzuela R, Tapia G, Varela P, Videla LA, Fernández V. Protection against in vivo liver ischemia-reperfusion injury by n-3 long-chain polyunsaturated fatty acids in the rat. Free Radic Res. 2010;44:854-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Mager DR, Wykes LJ, Roberts EA, Ball RO, Pencharz PB. Effect of orthotopic liver transplantation (OLT) on branched-chain amino acid requirement. Pediatr Res. 2006;59:829-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |