Published online Oct 21, 2012. doi: 10.3748/wjg.v18.i39.5601
Revised: June 27, 2012
Accepted: July 9, 2012
Published online: October 21, 2012
AIM: To explore differences in biochemical indices between neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD) and that with other etiologies.
METHODS: Patients under 6 mo of age who were referred for investigation of conjugated hyperbilirubinaemia from June 2003 to December 2010 were eligible for this study. After excluding diseases affecting the extrahepatic biliary system, all patients were screened for the two most common SLC25A13 mutations; the coding exons of the entire SLC25A13 gene was sequenced and Western blotting of citrin protein performed in selected cases. Patients in whom homozygous or compound heterozygous SLC25A13 mutation and/or absence of normal citrin protein was detected were defined as having NICCD. Cases in which no specific etiological factor could be ascertained after a comprehensive conjugated hyperbilirubinaemia work-up were defined as idiopathic neonatal cholestasis (INC). Thirty-two NICCD patients, 250 INC patients, and 39 infants with cholangiography-confirmed biliary atresia (BA) were enrolled. Laboratory values at their first visit were abstracted from medical files and compared.
RESULTS: Compared with BA and INC patients, the NICCD patients had significantly higher levels of total bile acid (TBA) [all measures are expressed as median (inter-quartile range): 178.0 (111.2-236.4) μmol/L in NICCD vs 112.0 (84.9-153.9) μmol/L in BA and 103.0 (70.9-135.3) μmol/L in INC, P = 0.0001]. The NICCD patients had significantly lower direct bilirubin [D-Bil 59.6 (43.1-90.9) μmol/L in NICCD vs 134.0 (115.9-151.2) μmol/L in BA and 87.3 (63.0-123.6) μmol/L in INC, P = 0.0001]; alanine aminotransferase [ALT 34.0 (23.0-55.0) U/L in NICCD vs 108.0 (62.0-199.0) U/L in BA and 84.5 (46.0-166.0) U/L in INC, P = 0.0001]; aspartate aminotransferase [AST 74.0 (53.5-150.0) U/L in NICCD vs 153.0 (115.0-239.0) U/L in BA and 130.5 (81.0-223.0) U/L in INC, P = 0.0006]; albumin [34.9 (30.7-38.2) g/L in NICCD vs 38.4 (36.3-42.2) g/L in BA and 39.9 (37.0-42.3) g/L in INC, P = 0.0001]; glucose [3.2 (2.0-4.4) mmol/L in NICCD vs 4.1 (3.4-5.1) mmol/L in BA and 4.0 (3.4-4.6) mmol/L in INC, P = 0.0014] and total cholesterol [TCH 3.33 (2.97-4.00) mmol/L in NICCD vs 4.57 (3.81-5.26) mmol/L in BA and 4.00 (3.24-4.74) mmol/L in INC, P = 0.0155] levels. The D-Bil to total bilirubin (T-Bil) ratio was significantly lower in NICCD patients [all measures are expressed as median (inter-quartile range): 0.54 (0.40-0.74)] than that in BA patients [0.77 (0.72-0.81), P = 0.001] and that in INC patients [0.74 (0.59-0.80), P = 0.0045]. A much higher AST/ALT ratio was found in NICCD patients [2.46 (1.95-3.63)] compared to BA patients [1.38 (0.94-1.97), P = 0.0001] and INC patients [1.48 (1.10-2.26), P = 0.0001]. NICCD patients had significantly higher TBA/D-Bil ratio [3.36 (1.98-4.43) vs 0.85 (0.72-1.09) in BA patients and 1.04 (0.92-1.14) in INC patients, P = 0.0001], and TBA/TCH ratio [60.7 (32.4-70.9) vs 24.7 (19.8-30.2) in BA patients and 24.2 (21.4-26.9) in INC patients, P = 0.0001] compared to the BA and INC groups.
CONCLUSION: NICCD has significantly different biochemical indices from BA or INC. TBA excretion in NICCD appeared to be more severely disturbed than that of bilirubin and cholesterol.
- Citation: Wang JS, Wang XH, Zheng YJ, Fu HY, Chen R, Lu Y, Fang LJ, Saheki T, Kobayashi K. Biochemical characteristics of neonatal cholestasis induced by citrin deficiency. World J Gastroenterol 2012; 18(39): 5601-5607
- URL: https://www.wjgnet.com/1007-9327/full/v18/i39/5601.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i39.5601
Citrin deficiency, caused by mutations in the SLC25A13 gene on chromosome 7q21.3, is an autosomal recessive disease that was first discovered in Japan and thereafter identified worldwide[1-8]. At least two main phenotypes of citrin deficiency have been established: neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD, OMIM #605814)[9-11] and adult-onset type II citrullinemia (CTLN2, OMIM #603471)[1,12]. The clinical features and diagnostic criteria of CTLN2 have been well established, but those of NICCD have not yet been established.
Children with NICCD usually have transient intrahepatic cholestasis that disappears by the age of 1 year with appropriate management[13]. However, some patients need liver transplantation or may die from the disease during infancy[14-17]. Others may develop severe CTLN2 symptoms unexpectedly one to several decades later[13]. Prompt detection and specific lactose-free and/or medium-chain triglyceride formula may contribute to the avoidance of a complicated course in the NICCD phase. However, the prompt diagnosis of NICCD is still a challenge because the clinical features of cholestasis induced by citrin deficiency are presently not fully understood[18].
Some biochemical indices, including total bilirubin (T-Bil), direct bilirubin (D-Bil), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyltranspeptidase (GGT), and α-fetoprotein (AFP) have been analyzed or compared between patients with NICCD, biliary atresia (BA) or idiopathic neonatal cholestasis (INC), with significant differences found between them[4]. However, comparisons of serum total bile acid (TBA) and total cholesterol (TCH) levels between different causes of neonatal cholestasis has rarely been reported before[19].
Bilirubin, bile acids and cholesterol are all mainly physiologically excreted by the hepatobiliary system. Usually, the blood levels of all these compounds increase in the event of blockage of the extrahepatic biliary system. However, they may be affected to different extents in intrahepatic cholestasis with different etiology. For instance, blood TCH and TBA levels are usually elevated significantly in Alagille syndrome, even in cases in which the bilirubin level is only mildly elevated; however, the blood TCH level is usually normal in cases of progressive familial intrahepatic cholestasis type 1 or 2 despite significant elevation of blood TBA and bilirubin levels. Therefore, comparison of these biochemical indices in neonatal cholestasis cases with different etiologies will enable better characterization of the biochemical changes of the disease and may help elucidate the mechanism of cholestasis caused by citrin deficiency.
Therefore, the aims of this study were to explore the differences in biochemical indices, including D-Bil, TBA, TCH and their ratios between cholestasis with different etiologies, and to explore the mechanism of cholestasis caused by citrin deficiency.
Patients under 6 mo of age who were referred to the Children’s Hospital of Fudan University, a tertiary referral hospital and primary specialized paediatric hospital in Eastern China, from June 2003 to December 2010, for investigation of conjugated hyperbilirubinaemia were eligible for this study. Conjugated hyperbilirubinemia was defined as serum T-Bil levels > 75 μmol/L, with a conjugated fraction accounting for > 20% of the total, or having conjugated bilirubin levels > 17 μmol/L with serum T-Bil levels < 75 μmol/L[20]. Patients who had obvious extrahepatic abnormalities or prolonged prothrombin time that could not be corrected by parenteral administration of vitamin K1 were excluded. Subjects who satisfied the above criteria as well as the following specific criteria for each of the three groups were included.
BA group: Patients with neonatal cholestasis in whom no isotope excretion was demonstrated by hepatobiliary iminodiacetic acid (HIDA) scintigraphy, and in whom diagnosis of BA was confirmed by laparoscopic or laparotomic cholangiography were eligible for this group.
INC group: Intrahepatic cholestasis was defined as conjugated hyperbilirubinemia following the exclusion of diseases affecting the extrahepatic biliary system (Table 1) by imaging of the hepatobiliary system. The imaging procedures included an ultrasound scan and HIDA scintigraphy in each case and laparotomic/laparoscopic cholangiography in selected cases. Idiopathic neonatal cholestasis (INC) was defined when no specific etiological factor (Table 1) could be ascertained after a comprehensive conjugated hyperbilirubinemia test[21]. Patients with at least single-allele mutation of SLC25A13 gene were excluded from this group as well.
| Affecting bile duct | Infectious | Metabolic | Others |
| Biliary atresia | Herpes viruses | α-1 antitrypsin deficiency | Endocrinological |
| Choledochal cyst | Rubella virus | Neonatal iron storage disease | Hypothyroidism |
| Cholelithiasis | Enteroviruses | Amino acid disorders | Panhypopituitarism/septo-optic dysplasia |
| Inspissated bile | Hepatitis viruses | Tyrosinemia | Genetic |
| Tumor | Human immunodeficiency virus | Hypermethioninemia | ATP8B1 deficiency |
| Hemangioma | Syphilis | Mevalonate kinase dificiency | ABCB11 deficiency |
| Bile duct stenosis/stricture/perforation | Toxoplasmosis | Glucogen storage diseases | ABCB4 deficiency |
| Neonatal sclerosing cholangitis | Bacterial sepsis | Gaucher disease | Bile acid synthetic defects |
| Caroli disease | Urinary tract infections | Niemann–Pick disease | Neonatal Dubin–Johnson syndrome |
| Alagille syndrome | Wolman disease | Various trisomies | |
| Zellweger syndrome | Arghrogryposis | ||
| Infantile Refsum disease | Hematological | ||
| Mitochondrial disorders | Hemophagocytic lymphohistiocytosis | ||
| Urea cycle disorders | Langerhans cell histiocytosis | ||
| Miscellaneous drug effects | |||
| Total parenteral nutrition |
NICCD group: The strategy of testing for SLC25A13 gene mutations in intrahepatic cholestatic infants had been reported previously[22,23]. All intrahepatic cholestasis infants with unknown causes were screened for the two most common mutations of the SLC25A13 gene in Chinese, 851del4 and 1638ins23. For patients with various aminoacidemia or patients with only single-allele mutation who were found by the above screening method, the entire 18 coding exons together with the flanking sequence of the SLC25A13 gene were amplified by polymerase chain reaction and directly sequenced. Western blotting analysis of citrin protein was performed on patients with biopsied liver specimens available. Only patients in whom homozygous or compound heterozygous SLC25A13 gene mutation and/or absence of normal citrin protein were demonstrated, for whom a definite diagnosis of citrin deficiency could be made, were regarded as NICCD patients in this study. Patients with a probable diagnosis of citrin deficiency, that is, in whom there was only a heterozygous SLC25A13 gene mutation and in whom absence of normal citrin protein could not be demonstrated by Western blotting were excluded.
The medical files of the patients who satisfied the above inclusion and exclusion criteria were reviewed following the approval of the Institute’s Ethics Review Committee. Sex, birth weight, gestation age or term/preterm, age at which conjugated jaundice was first noticed, and the biochemical indices at presentation, were abstracted. Liver function tests and other routine laboratory data were obtained using standard methods.
Statistical analysis was performed using Stata/SE 10.0 for Windows (StataCorp LP, College Station, TX, United States of America). The descriptive data of the quantitative variables were reported in box-whisker plots and compared using Kruskal-Wallis rank tests among the three groups. For results with overall statistical significance, a Mann-Whitney test with a Bonferroni correction was further performed to test the medians between a series of pairwise groups. All P values were two-sided. Results were considered statistically significant at the 0.05 level.
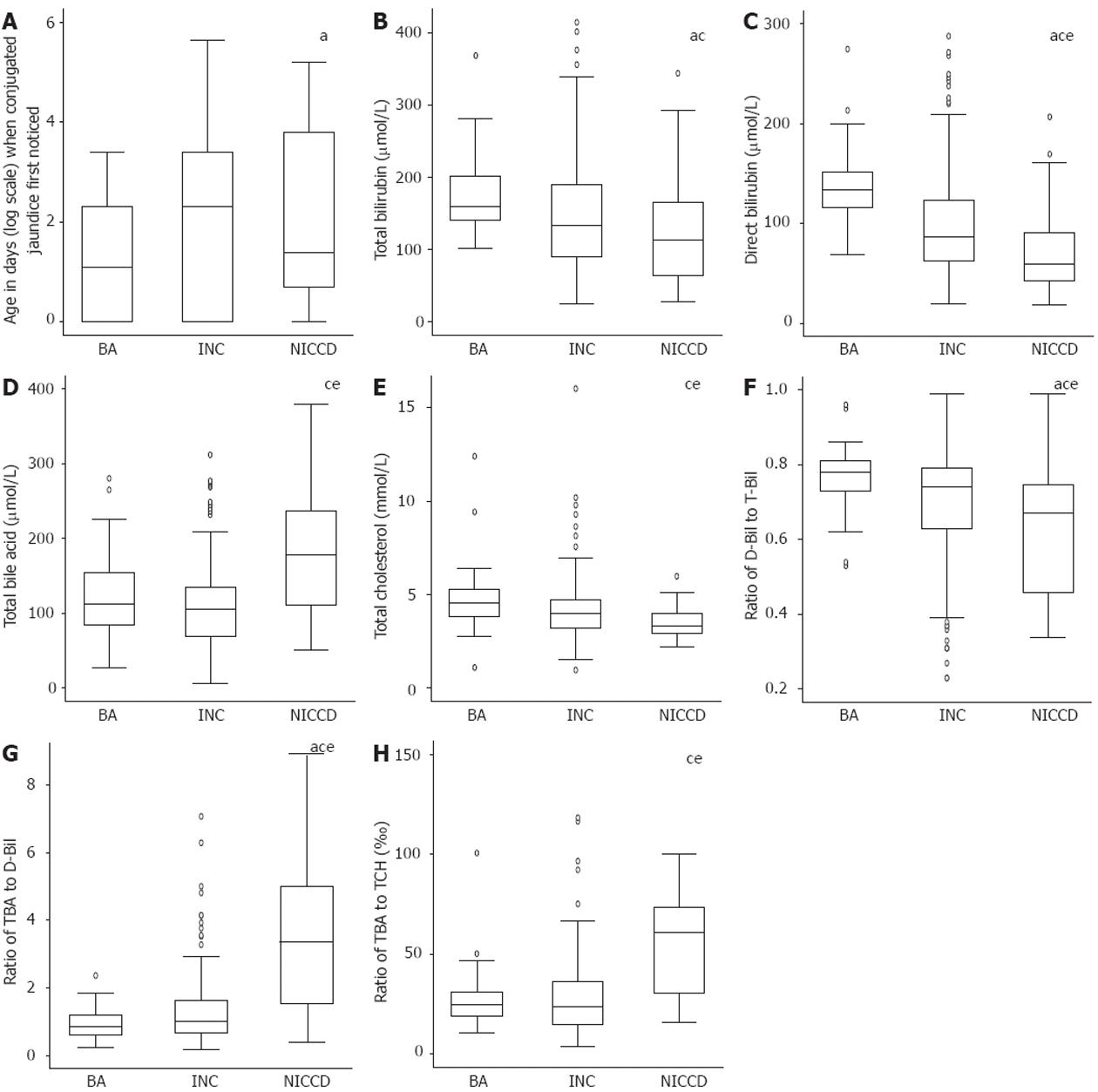
In total, 32 patients (19 male and 13 female) with a definite diagnosis of citrin deficiency were included in the NICCD group. Thirty-nine patients (24 male and 15 female) were included in the BA group. Two hundred and fifty patients (174 male and 76 female) were included in the INC group. The birth weight and the days at which conjugated jaundice was first noticed in the three groups are illustrated in Figure 1 or Table 2. The median birth weight was lowest in the NICCD group, but the difference did not reach statistical significance (Table 2). Conjugated jaundice was noticed earlier in the BA group compared with the INC group (P < 0.05, Figure 1A).
| Reference range and unit | BA (n = 39) | INC (n = 39) | NICCD (n = 32) | ||||
| Median | Inter-quartile | Median | Inter-quartile | Median | Inter-quartile | ||
| Birth weight | 2.5-4.0 kg | 3.2 | 2.9-3.8 | 3.1 | 2.8-3.5 | 2.9 | 2.4-3.4 |
| Biochemical indices | |||||||
| T-Bilac | 2-20 mmol/L | 159.5 | 140.2-201.4 | 133.8 | 90.0-190.4 | 112.7 | 64.4-165.4 |
| D-Bilace | 0-6 mmol/L | 134 | 115.9-151.2 | 87.3 | 63.0-123.6 | 59.6 | 43.1-90.9 |
| ALTce | < 40 IU/L | 108 | 62.0-199.0 | 84.5 | 46.0-166.0 | 34 | 23.0-55.0 |
| ASTce | < 40 IU/L | 153 | 115.0-239.0 | 130.5 | 81.0-223.0 | 74 | 53.5-150.0 |
| GGTac | < 50 IU/L | 558 | 300.0-1086.0 | 155 | 91.0-294.0 | 187.5 | 136.0-253.0 |
| TBAce | < 40 mmol/L | 112 | 84.9-153.9 | 103 | 70.9-135.3 | 177.9 | 111.2-236.4 |
| Total proteince | 55-78 g/L | 57.4 | 55.3-63.1 | 57.2 | 52.5-62.8 | 48.5 | 44.5-53.9 |
| Albumince | 35-55 g/L | 38.4 | 36.3-42.2 | 39.9 | 37.0-42.3 | 34.9 | 30.7-38.2 |
| Glucosece | 3.9-5.9 mmol/L | 4.1 | 3.4-5.1 | 4 | 3.4-4.6 | 3.2 | 2.0-4.4 |
| TCHce | 3.12-5.20 mmol/L | 4.57 | 3.81-5.26 | 4 | 3.24-4.74 | 3.33 | 2.97-4.00 |
| Ratios | |||||||
| D-Bil/T-Bilace | 0.77 | 0.72-0.81 | 0.74 | 0.59-0.80 | 0.54 | 0.40-0.74 | |
| AST/ALTce | 1.38 | 0.94-1.97 | 1.48 | 1.10-2.66 | 2.46 | 1.95-3.63 | |
| TBA/D-Bilace | 0.85 | 0.72-1.09 | 1.04 | 0.92-1.14 | 3.36 | 1.98-4.43 | |
| D-Bil/TCHac | 30.2 | 22.8-34.0 | 21.5 | 16.7-31.2 | 18.7 | 13.9-26.6 | |
| TBA/TCHce | 24.7 | 19.8-30.2 | 24.2 | 21.4-26.9 | 60.7 | 32.4-70.9 | |
The biochemical data of the three groups were compared (Figure 1B-E, Table 2). The NICCD group had significantly lower ALT, AST, total protein, albumin, and glucose levels compared with the BA and INC groups, suggesting that the synthetic function and glucose metabolism were more severely damaged in the NICCD group than in the other two groups. The NICCD group also had significantly higher TBA and lower D-Bil and cholesterol levels compared with the BA and INC groups, indicating that the excretion of bile acids, D-Bil and cholesterol might be affected differently in NICCD patients. Significantly lower T-Bil and GGT levels were noticed in the NICCD group only when compared with the BA group.
To compare further the different biochemical indices, a series of ratios was calculated. The highest ratio of D-Bil to T-Bil was found in the BA group and the lowest in the NICCD group (Table 2, Figure 1F). A much higher AST/ALT ratio was found in the NICCD group compared to the INC and BA groups (Table 2).
The ratios between D-Bil, bile acids and cholesterol were also compared (Table 2). The ratio of serum TBA to D-Bil was significantly higher in the NICCD group than the ratios in the BA and INC groups (Figure 1G, P < 0.05). Significant differences were also found for the ratio of TBA to TCH between the NICCD and BA groups and between the NICCD and INC groups (Figure 1H, P < 0.05).
Citrin deficiency is one of the most common classical inborn errors of metabolism of amino acids, organic acids and fatty acid oxidation in Eastern Asia, including China[24]. The biochemical characteristics and mechanism of cholestasis caused by citrin deficiency are still not fully understood. Although differences in some indices among patients with BA, INC and NICCD have been reported previously, the very small sample sizes of the studies precluded a definite conclusion[4,19,23]. In the present study, the cohorts of NICCD, INC and BA had numbers large enough to test previous findings. Additionally, by comparing the elevation of D-Bil, TBA and cholesterol levels and the ratios of these compounds, it was found that the excretion of bile acids appeared to be more severely affected in NICCD than in BA and INC.
A previous study with a small number of subjects demonstrated that patients with cholestasis caused by citrin deficiency had lower ALT and AST levels and higher AST to ALT ratios compared to those with BA or idiopathic neonatal hepatitis[25]. Low albumin and glucose levels were also associated with NICCD in a previous case series[26,27]. In the present study, those findings were confirmed. Previous studies also showed that patients with NICCD had lower birth weight compared to normal controls or to the national standard. In our study, although a lower median birth weight in the NICCD group was noticed, the differences did not reach statistical significance compared to patients with BA or INC. This could be explained by the different control groups (normal control or national standard used in previous studies vs patients with cholestasis of other causes) and the large difference observed within the NICCD group in this study.
The serum TBA level in NICCD has previously been compared with that in BA and INC in a study that had very few subjects[19]. In the present study, the serum level of TBA as well as the ratio of serum TBA to D-Bil and cholesterol levels was compared. In BA, we can suppose that excretion of D-Bil, bile acids and cholesterol is affected to the same extent in consideration of complete blockage of the biliary system. If the ratio of TBA to D-Bil in the BA group was taken as the reference value, the median for INC was found to be 1.22 (1.04/0.85) times higher and that of NICCD 3.95 (3.36/0.85) times higher. For the ratio of TBA to cholesterol, if the median value of the BA group was taken as a standard, the median in the INC group was nearly the same as the standard but that in the NICCD group was 2.46 (60.7/24.7) times higher. These results indicate that the excretion of bile acids is much more severely affected than the excretion of bilirubin and cholesterol in NICCD patients. As a consequence, we may speculate that the failure to excrete bile acids from hepatocytes to the canalicula is the main mechanism of cholestasis caused by citrin deficiency.
The main limitation of this study was its retrospective nature. It could be argued that some biochemical indices were affected by the drugs that patients were taking. However, prior to the determination of a clear diagnosis, the management of patients had been similar in the three groups; therefore, the patients in the different groups would have been affected by these variables in the same way. Another measure that was used to avoid sample bias was using the first available laboratory data obtained when patients were referred to us. Although significant differences in TBA and TBA ratios were found between the NICCD and other two groups, no cut-off levels can be presented at this time.
Citrin deficiency is one of the most common metabolic disorders in Eastern Asia. It has at least two main phenotypes: neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD) and adult-onset type II citrullinemia. The clinical features of and the mechanism of cholestasis in NICCD have yet to be established.
Some biochemical indices of patients with NICCD have been compared to those of patients with biliary atresia (BA) and of patients with idiopathic neonatal cholestasis (INC). Comparison of these biochemical indices in neonatal cholestasis cases with different etiologies will better characterize the biochemical changes of the disease and may further the understanding of the mechanism of cholestasis caused by citrin deficiency.
Apart from confirming previous findings that NICCD patients had significantly lower alanine aminotransferase (ALT) level, lower direct bilirubin (D-Bil) to total bilirubin ratio, and significantly higher aspartate aminotransferase to ALT ratio compared to the BA and INC patients, this study specifically compared the serum level of total bile acid (TBA) and its ratio to D-Bil and cholesterol, and found that NICCD patients had significantly higher TBA levels as well as higher TBA to D-Bil and TBA to cholesterol ratios than patients with BA and INC.
The excretion of TBA appears to be much more severely disturbed than that of D-Bil and cholesterol in cholestasis caused by citrin deficiency. Further study of this condition will help elucidate the mechanism of cholestasis in NICCD, and the ratios could be further developed as indices for the differential diagnosis of neonatal cholestasis.
The authors present an interesting retrospective study comparing liver specific biochemical parameters in different groups of infants with cholestasis.
Peer reviewer: Dr. Stefan Wirth, Professor, Children’s Hospital, Heusnerstt. 40, 42349 Wuppertal, Germany
S- Editor Lv S L- Editor Kerr C E- Editor Lu YJ
| 1. | Kobayashi K, Sinasac DS, Iijima M, Boright AP, Begum L, Lee JR, Yasuda T, Ikeda S, Hirano R, Terazono H. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat Genet. 1999;22:159-163. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Lu YB, Kobayashi K, Ushikai M, Tabata A, Iijima M, Li MX, Lei L, Kawabe K, Taura S, Yang Y. Frequency and distribution in East Asia of 12 mutations identified in the SLC25A13 gene of Japanese patients with citrin deficiency. J Hum Genet. 2005;50:338-346. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Song YZ, Hao H, Ushikai M, Liu GS, Xiao X, Saheki T, Kobayashi K, Wang ZN. [A difficult and complicated case study: neonatal intrahepatic cholestasis caused by citrin deficiency]. Zhongguo Dangdai Erke Zazhi. 2006;8:125-128. [PubMed] [Cited in This Article: ] |
| 4. | Yeh JN, Jeng YM, Chen HL, Ni YH, Hwu WL, Chang MH. Hepatic steatosis and neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD) in Taiwanese infants. J Pediatr. 2006;148:642-646. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Ko JS, Song JH, Park SS, Seo JK. Neonatal intrahepatic cholestasis caused by citrin deficiency in Korean infants. J Korean Med Sci. 2007;22:952-956. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Thong MK, Boey CC, Sheng JS, Ushikai M, Kobayashi K. Neonatal intrahepatic cholestasis caused by citrin deficiency in two Malaysian siblings: outcome at one year of life. Singapore Med J. 2010;51:e12-e14. [PubMed] [Cited in This Article: ] |
| 7. | Hutchin T, Preece MA, Hendriksz C, Chakrapani A, McClelland V, Okumura F, Song YZ, Iijima M, Kobayashi K, Saheki T. Neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD) as a cause of liver disease in infants in the UK. J Inherit Metab Dis. 2009;Jun 11; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Dimmock D, Maranda B, Dionisi-Vici C, Wang J, Kleppe S, Fiermonte G, Bai R, Hainline B, Hamosh A, O'Brien WE. Citrin deficiency, a perplexing global disorder. Mol Genet Metab. 2009;96:44-49. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Ohura T, Kobayashi K, Tazawa Y, Nishi I, Abukawa D, Sakamoto O, Iinuma K, Saheki T. Neonatal presentation of adult-onset type II citrullinemia. Hum Genet. 2001;108:87-90. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Naito E, Ito M, Matsuura S, Yokota T, Ogawa Y, Kitamura S, Kobayashi K, Saheki T, Nishimura Y, Sakura N. Type II citrullinaemia (citrin deficiency) in a neonate with hypergalactosaemia detected by mass screening. J Inherit Metab Dis. 2002;25:71-76. [PubMed] [Cited in This Article: ] |
| 11. | Yamaguchi N, Kobayashi K, Yasuda T, Nishi I, Iijima M, Nakagawa M, Osame M, Kondo I, Saheki T. Screening of SLC25A13 mutations in early and late onset patients with citrin deficiency and in the Japanese population: Identification of two novel mutations and establishment of multiple DNA diagnosis methods for nine mutations. Hum Mutat. 2002;19:122-130. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Yasuda T, Yamaguchi N, Kobayashi K, Nishi I, Horinouchi H, Jalil MA, Li MX, Ushikai M, Iijima M, Kondo I. Identification of two novel mutations in the SLC25A13 gene and detection of seven mutations in 102 patients with adult-onset type II citrullinemia. Hum Genet. 2000;107:537-545. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Ohura T, Kobayashi K, Tazawa Y, Abukawa D, Sakamoto O, Tsuchiya S, Saheki T. Clinical pictures of 75 patients with neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD). J Inherit Metab Dis. 2007;30:139-144. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Tamamori A, Okano Y, Ozaki H, Fujimoto A, Kajiwara M, Fukuda K, Kobayashi K, Saheki T, Tagami Y, Yamano T. Neonatal intrahepatic cholestasis caused by citrin deficiency: severe hepatic dysfunction in an infant requiring liver transplantation. Eur J Pediatr. 2002;161:609-613. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Shigeta T, Kasahara M, Kimura T, Fukuda A, Sasaki K, Arai K, Nakagawa A, Nakagawa S, Kobayashi K, Soneda S. Liver transplantation for an infant with neonatal intrahepatic cholestasis caused by citrin deficiency using heterozygote living donor. Pediatr Transplant. 2010;14:E86-E88. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Xing YZ, Qiu WJ, Ye J, Han LS, Xu SS, Zhang HW, Gao XL, Wang Y, Gu XF. [Studies on the clinical manifestation and SLC25A13 gene mutation of Chinese patients with neonatal intrahepatic cholestasis caused by citrin deficiency]. Zhonghua Yixue Yichuanxue Zazhi. 2010;27:180-185. [PubMed] [Cited in This Article: ] |
| 17. | Song YZ, Deng M, Chen FP, Wen F, Guo L, Cao SL, Gong J, Xu H, Jiang GY, Zhong L. Genotypic and phenotypic features of citrin deficiency: five-year experience in a Chinese pediatric center. Int J Mol Med. 2011;28:33-40. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Dimmock D, Kobayashi K, Iijima M, Tabata A, Wong LJ, Saheki T, Lee B, Scaglia F. Citrin deficiency: a novel cause of failure to thrive that responds to a high-protein, low-carbohydrate diet. Pediatrics. 2007;119:e773-e777. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Tazawa Y, Abukawa D, Sakamoto O, Nagata I, Murakami J, Iizuka T, Okamoto M, Kimura A, Kurosawa T, Iinuma K. A possible mechanism of neonatal intrahepatic cholestasis caused by citrin deficiency. Hepatol Res. 2005;31:168-171. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | De Bruyne R, Van Biervliet S, Vande Velde S, Van Winckel M. Clinical practice: neonatal cholestasis. Eur J Pediatr. 2011;170:279-284. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Wang JS, Wang ZL, Wang XH, Zhu QR, Zheng S. The prognostic value of serum gamma glutamyltransferase activity in Chinese infants with previously diagnosed idiopathic neonatal hepatitis. HK J Pediatr. 2008;13:39-45. [Cited in This Article: ] |
| 22. | Fu HY, Zhang SR, Wang XH, Saheki T, Kobayashi K, Wang JS. The mutation spectrum of the SLC25A13 gene in Chinese infants with intrahepatic cholestasis and aminoacidemia. J Gastroenterol. 2011;46:510-518. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Fu HY, Zhang SR, Yu H, Wang XH, Zhu QR, Wang JS. Most common SLC25A13 mutation in 400 Chinese infants with intrahepatic cholestasis. World J Gastroenterol. 2010;16:2278-2282. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Lee HC, Mak CM, Lam CW, Yuen YP, Chan AO, Shek CC, Siu TS, Lai CK, Ching CK, Siu WK. Analysis of inborn errors of metabolism: disease spectrum for expanded newborn screening in Hong Kong. Chin Med J (Engl). 2011;124:983-989. [PubMed] [Cited in This Article: ] |
| 25. | Chen HW, Chen HL, Ni YH, Lee NC, Chien YH, Hwu WL, Huang YT, Chiu PC, Chang MH. Chubby face and the biochemical parameters for the early diagnosis of neonatal intrahepatic cholestasis caused by citrin deficiency. J Pediatr Gastroenterol Nutr. 2008;47:187-192. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Tazawa Y, Kobayashi K, Abukawa D, Nagata I, Maisawa S, Sumazaki R, Iizuka T, Hosoda Y, Okamoto M, Murakami J. Clinical heterogeneity of neonatal intrahepatic cholestasis caused by citrin deficiency: case reports from 16 patients. Mol Genet Metab. 2004;83:213-219. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Kimura A, Kage M, Nagata I, Mushiake S, Ohura T, Tazawa Y, Maisawa S, Tomomasa T, Abukawa D, Okano Y. Histological findings in the livers of patients with neonatal intrahepatic cholestasis caused by citrin deficiency. Hepatol Res. 2010;40:295-303. [PubMed] [DOI] [Cited in This Article: ] |