Published online Oct 7, 2006. doi: 10.3748/wjg.v12.i37.5987
Revised: August 20, 2005
Accepted: November 10, 2005
Published online: October 7, 2006
AIM: To evaluate the intestinal motility changes evoked by 8 bacterial strains belonging to Bifidobacterium, Lactobacillus and Streptococcus genera within the probiotic preparation VSL#3.
METHODS: Ileum and proximal colon segments isolated from guinea-pigs were used as a study model. Entire cells and cell fractions (cell debris, cell wall fraction, cytoplasmatic fraction, proteinaceous and non-proteinaceous cytoplasmatic components) of VSL#3 strains and, as controls, Escherichia coli, Salmonella aboni and Bacillus licheniformis were tested in this in vitro model.
RESULTS: Among the bacterial cell fractions tested, only the cytoplasmatic fraction modified intestinal motility. Lactobacillus strains stimulated the contraction of ileum segment, whereas all probiotic strains tested induced proximal colon relaxation response. The non-proteinaceous cytoplasmatic components were responsible for the colon relaxation.
CONCLUSION: The results obtained in this study suggest that the proximal colon relaxation activity showed by the probiotic bacteria could be one of the possible mechanisms of action by which probiotics exert their positive effects in regulating intestinal motility.
- Citation: Massi M, Ioan P, Budriesi R, Chiarini A, Vitali B, Lammers KM, Gionchetti P, Campieri M, Lembo A, Brigidi P. Effects of probiotic bacteria on gastrointestinal motility in guinea-pig isolated tissue. World J Gastroenterol 2006; 12(37): 5987-5994
- URL: https://www.wjgnet.com/1007-9327/full/v12/i37/5987.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i37.5987
Probiotics are viable microbial cells that upon ingestion in specific numbers appear to have beneficial effects on the health and well-being of the host, beyond inherent basic nutrition[1]. These health-promoting effects are predominantly related to reinforcement of the intestinal mucosal barrier against enteropathogens’ colonization, immunostimulation and immunomodulation, anticarcino-genic and antimutagenic activities, improvement of lactose utilization, and reduction of serum cholesterol[2].
Most probiotics are bacteria members of the genera Lactobacillus and Bifidobacterium, which represent important components of human gastrointestinal flora. However, other nonpathogenic bacteria, such as Streptococcus, some strains of Eschericha coli and Enterococcus faecium, and yeasts, such as Saccharomyces boulardii, have been used in probiotic preparations[3].
Experimental and clinical studies support the use of probiotics in the treatment of intestinal disorders such as infectious diarrhea[4-7], antibiotic diarrhea[8-10], traveller’s diarrhea[11-13], irritable bowel syndrome or functional diarrhea[14-18] and inflammatory bowel disease[19-23]. In recent double-blind placebo controlled trials the efficacy of the probiotic preparation VSL#3 has been shown as maintenance treatment and prophylactic therapy in patients with diarrhea-predominant irritable bowel syndrome and pouchitis[18,22-24]. VSL#3 (VSL Pharmaceuticals, Ft. Lauderdale, FL, USA) contains a mixture of eight different bacterial species at very high concentrations (450 billions/sachet of viable lyophilized bacteria). The preparation consists of three strains of bifidobacteria (Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis), four strains of lactobacilli (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus plantarum) and one strain of Streptococcus thermophilus.
To the best of our knowledge, there are no experi-mental data evaluating the effects of probiotic bacteria on the intestinal motility. Functional assays could represent useful tools to investigate the effects evoked by the entire probiotic cells and/or their fraction on different segments of the intestinal tissue.
The aim of this study was to evaluate the effect of the eight strains within the probiotic preparation VSL#3[18,21-23] on intestinal motility using ileum and proximal colon segments isolated from guinea-pigs. Entire bacterial cells, cell fractions of VSL#3 strains, and, as controls, Escherichia coli, Salmonella aboni and Bacillus licheniformis, were tested in this in vitro model.
The following bacterial strains were used in this study: Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus plantarum and Streptococcus thermophilus.
Bifidobacterium and Lactobacillus strains were grown anaerobically (Anaerobic System, Model 2028, Forma Scientific, Marietta, OH, USA) in MRS medium (Difco, Detroit, MI, USA) supplemented with 0.05% L-cysteine hydrochloride monohydrate (Merck, Darmstadt, Germany), at 37°C. S. thermophilus was cultured anaerobically in M17 medium (Difco) at 37°C. Salmonella aboni NCTC 6017, Escherichia coli ATCC 11105 and Bacillus licheniformis BGSC 5A24 were used as controls and grown aerobically in LB medium (Difco) at 37°C.
Ten milliliter of the bacterial mid log cultures, counted by plating technique on the previously mentioned media, was collected by centrifugation (5000 × g at 4°C for 5 min), washed, and resuspended in 1 mL of De Jalon buffer (155 mmol/L NaCl, 5.6 mmol/L KCl, 0.5 mmol/L CaCl2, 6.0 mmol/L NaHCO3, 2.8 mmol/L glucose)[25], obtaining a final bacterial concentration of 1 × 109-1 × 1010 colony forming units (CFUs/mL). Subsequently the bacterial suspensions were sonicated (Bronson Sonifier W-250, Heineman, Schwäbisch, Germany) at power level 2 at 20% duty for 8 min and centrifuged at 8000 × g for 30 min to separate cell debris from crude extract. Cell debris was resuspended in 1 mL of De Jalon buffer. The crude extract was ultracentrifuged at 250 000 × g at 4°C for 2 h: the supernatant represents the cytoplasmatic fraction while the pellet, resuspended in 1 mL of 50 mmol/L TRIS.HCl pH 7.6, represents the fraction enriched in membrane proteins.
In order to obtain the bacterial cell wall fraction, bacterial cultures were centrifuged at 5000 × g at 4°C for 5 min, washed in 50 mmol/L Tris HCl pH 7.6, resuspended in 1 mL of protoplast buffer [50 mmol/L Tris HCl pH 7.6, 1 mol/L sucrose, 50 mL of completeTM protease inhibitor (Roche, Mannheim, Germany), 15 mg/mL lysozyme] and incubated at 37°C for 20 min. The supernatant was recovered by centrifuging at 5000 × g at 4°C for 5 min.
Proteinaceous components were precipitated by addition of 9 volumes of acetone:HCl (10:0.1) to one volume of the cytoplasmatic fraction and collected by centrifuging at 12 000 × g at 4°C for 5 min. Supernatant (non-proteinaceous fraction) and protein pellet were dried on ice under vacuum for removing acetone and resuspended in the initial volume of 50 mmol/L TRIS.HCl pH 7.6. The very low value of pH (about 1) of the non-proteinaceous fraction was adjusted to a value of 7.4 to overcome the unspecific colonic contractile response due to the acid pH. Similarly, the cytoplasmatic fraction was incubated with proteinase K (500 mg/mL) at 50°C for 1 h for the enzymatic digestion of the proteins. All the bacterial fractions of cell debris (i.e., cell wall fraction, membrane proteins, cytoplasmatic fractions, proteinaceous and non-proteinaceous cytoplasmatic components) were aliquoted and stored at -80°C before to be used in the in vitro stimulation of ileum and proximal colon.
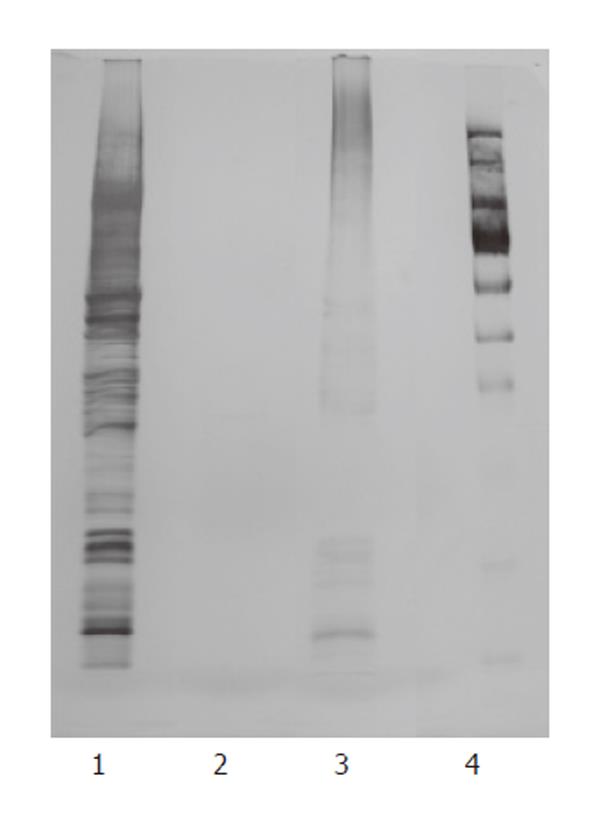
Ten μL of B. infantis cytoplasmatic fraction, non-proteinaceous fraction and cytoplasmatic fraction deprived of proteins by proteinase K digestion was analyzed by SDS-PAGE, as described by Laemmli[26], using a 12% polyacrylamide running gel. The gels were stained with silver nitrate.
Isolation of genomic DNA from pure cultures of the probiotic bacteria was performed as previously described[27]. In order to obtain the complete cell disruption, the method was slightly modified by prolonging the enzymatic lysis for 1 to 3 h and grinding with glass beads (150-212 μm, Sigma, St. Louis, MO, USA). Concentration and purity of all DNA preparations were determined by measuring OD260 absorbance and OD260/280 ratio, respectively. Only DNAs with an OD260/280 ratio > 1.8 were used.
Guinea-pigs of either sex (200-400 g) obtained from Charles River (Calco, Como, Italy) were used. The animals were housed according to the ECC Council Directive regarding the protection of animals used for experimental and other scientific purposes. All procedures followed the guidelines of The Animal Care and Use Committee of The University of Bologna (Bologna, Italy). The animals were sacrificed by cervical dislocation, and the organ (ileum or proximal colon) required was set up rapidly under a suitable resting tension in 15-mL organ baths containing appropriate physiological salt solution (PSS) consistently warmed (see below) and buffered to pH 7.4 by saturation with 950 mL/L O2 and 50 mL/L CO2 gas.
The terminal portion of ileum (3-4 cm near the ileo-caecal junction) was cleaned with Tyrode solution of the following composition: 118 mmol/L NaCl, 4.75 mmol/L KCl, 2.54 mmol/L CaCl2, 1.20 mmol/L MgSO4, 1.19 mmol/L KH2PO4·2H2O, 25 mmol/L NaHCO3, and 11 mmol/L glucose. The mesenteric tissue was removed. The ileum tissue was cut in two segments of 2-3 cm taken in the longitudinal direction along the intestinal wall. The segments were set up upright under 1-g tension in a jacketed tissue bath (15 mL, 37°C) containing Tyrode solution buffered to pH 7.4 by saturation with 950 mL/L O2 and 50 mL/L CO2 gas. Tissues were allowed to equilibrate for at least 30 min, during which time the bathing solution was changed every 10 min. After stabilization, the strips were challenged with 1 μmol/L carbachol (Sigma, Italy) to assess the responsive capacity of the tissue[28].
Starting approximately 1 cm distal from the caecocolonic junction, a segment of about 3 cm was excised, cleansed by rinsing it with De Jalon solution[25], and the mesenteric tissue was removed. The proximal colon segment was cut in two segments of about 1 cm each taken in the longitudinal direction along the intestinal wall. The segments were set up upright under 1-g tension at 37°C in a jacketed tissue bath (15 mL, 37°C) containing De Jalon solution buffered to pH 7.4 by saturation with 950 mL/L O2 and 50 mL/L CO2 gas. Tissues were allowed to equilibrate for at least 30 min during which time the bathing solution was changed every 10 min. After stabilization, the strips were challenged with 5 μmol/L 5-hydroxytryptamine (5-HT) (Sigma, Italy) in the presence of 1 μmol/L atropine (Sigma, Italy) to assess the responsive capacity of the tissue.
After stabilization and assessment of the responsive capacity of the tissue in the organ bath, concentration-response curves were constructed by cumulative addition of the above described bacterial preparations (1 to 1500 μL). Each successive addition of bacterial preparations was performed after the response to the previous addition reached its maximum level and remained steady. Longitudinal muscle contractions or relaxations were recorded isometrically by securing one end of the tissue segments to a tissue holder and the other end to a force displacement transducer (FT. 03, Grass Instruments, Quincy, MA) using Power Lab software (ADInstruments Pty Ltd, Castle Hill, Australia). Each tracing was obtained by using separate intestinal strips.
Experiments were performed in duplicate with tissue from the same animal, and mean values were recorded. All data are presented as mean ± SE (n = 3-5 for ileum and n = 5-7 for proximal colon). Differences between means were calculated with Student t-test and P-values < 0.05 were considered statistically significant[29-31].
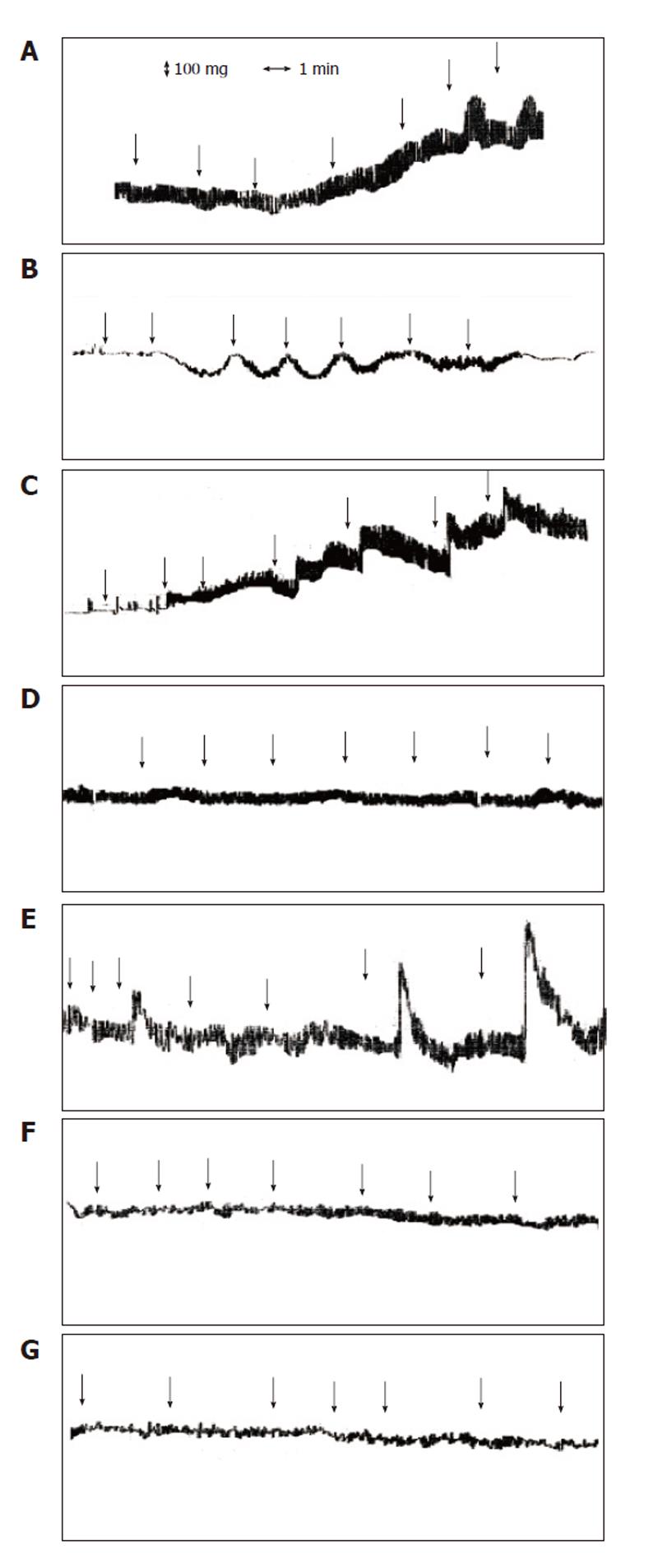
The motility response of the guinea-pig ileum segment was investigated with increasing concentrations of the following components of the probiotic VSL#3 mixture: live bacteria, bacterial cell debris, and crude extracts. VSL#3 live bacteria and cell debris did not modify the guinea-pig ileum motility (data not shown), whereas crude extracts dose-dependently increased both spontaneous phasic and tonic contractions of the ileum (Figure 1A). The effects on motility induced by crude extracts persisted even after adjusting the pH of the solution from around 5.0, as found in the crude extract, to pH 7.4 and were reversible as the basal tone returned to initial values after several washing steps (data not shown).
To identify the microbial genera within the VSL#3 cocktail responsible for the effects on motility in the guinea-pig ileum, crude extract mixtures of Bifidobacterium (B. longum, B. infantis, B. breve), Lactobacillus (L. acidophilus, L. casei, L. plantarum, L. bulgaricus), and Streptococcus thermophilus were individually tested. The following differences in the motility response by the crude extracts of the three probiotic genera were observed: (1) Lactobacillus strains induced a concentration-dependent contraction of the ileum, which was rapid in onset; (2) Bifidobacterium promoted a weak and transient stimulation of the ileum in a non concentration-dependent manner; (3) Streptococcus did not promote any significant response (Figures 1B-D).
The intestinal bacteria Salmonella aboni and Escherichia coli and the non-intestinal bacterium Bacillus licheniformis were used as controls to verify the specificity of the probiotic effects on gut motility (Figures 1E-G). Ileum motility was not affected by the exposure to E. coli and B. licheniformis crude extracts (Figures 1F, G), whereas S. aboni crude extracts were able to trigger the ileum contraction in a dose-dependent manner (Figure 1E). Furthermore, the amplitude of contraction induced by S. aboni was significantly higher than that promoted by the VSL#3 mixture and Lactobacillus crude extracts.
In order to verify the involvement of muscarinic receptors in the observed contraction response, the muscarinic antagonist atropine (1 μmol/L) was added to the organ bath. Atropine did not modify the motility response of ileal tissue exposed to all the bacterial crude extracts tested (data not shown).
The motility response of guinea-pig proximal colon segment was investigated using a similar protocol as with the guinea-pig ileum by adding identical concentrations of the bacterial cell preparations tested in the ileum stimulation study. Similar to the guinea-pig ileum, only the crude extracts of the probiotic VSL#3 mixture showed a significant effect on colon motility. However, the effect on the guinea-pig proximal colon was opposite to that observed in ileum (Figure 2A). In particular, the crude extract of the VSL#3 mixture elicited a rapid and sustained relaxation of the colon tissue, characterized by peaks in response to each addition, together with a progressive lowering of the basal tone. As previously carried out with the ileum, crude extract preparations at pH 5 and 7.4 were tested obtaining the same colon relaxation response (data not shown).
The VSL#3 extract mixtures of the genera Bifidoba-cterium, Lactobacillus, and S. thermophilus were assessed (Figures 2B-D). All the bacterial groups provoked a dose-dependent relaxation of the colon tissue, but no significant motility effect was observed by exposure to the control bacteria (Figures 2E-G). Clear colon relaxation effects were promoted by the crude extracts of Bifidobacterium strains and S. thermophilus and were quite similar to those induced by the crude extracts of the VSL#3 mixture (Figure 3). Lactobacillus strain’s crude extract showed a lower relaxation response. Washings of the colon segment exposed to successive additions of all crude extracts tested restored the basal tone of the tissue (data not shown). A further investigation was carried out with the crude extracts of VSL#3 B. infantis and L. casei strains, representative of Bifidobacterium and Lactobacillus genera. Colon relaxation patterns induced by these samples were identical to those obtained with the crude extracts of Bifidobacterium and Lactobacillus mixtures (data not shown).
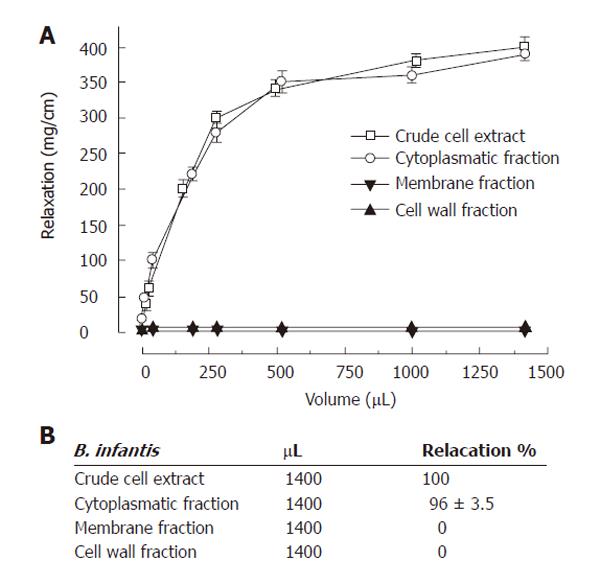
To confirm that bacterial cytoplasmatic components are mainly responsible for colon relaxation, a more refined fractioning of the B. infantis cells was performed by separating cell wall fraction, membrane proteins, and cytoplasmatic fraction. A concentration-response curve was constructed for each of these bacterial portions (Figure 4). Indeed, the cytoplasmatic fraction showed a sustained relaxation of the colon segment equal to that demonstrated by the B. infantis crude extract. As expected, the cell wall fraction and the membrane proteins did not produce any significant effect.
The cytoplasmatic fraction of B. infantis was further refined in proteinaceous and non-proteinaceous components. The absence of protein in the non-proteinaceous fraction was verified by SDS PAGE analysis (Figure 5). As reported in Figure 6, the effect on colon relaxation was induced by the non-proteinaceous components, which showed a motility response similar to that exerted by the cytoplasmatic fraction. The relaxation effect exerted by the non-proteinaceous components was confirmed by colon motility experiments carried out with a B. infantis cytoplasmatic fraction deprived of proteins by proteinase K digestion. This preparation, in which the enzymatic protein degradation was demonstrated by SDS PAGE analysis (Figure 5), evoked a significant colonic relaxation response similar to that obtained with the buffered non-proteinaceous preparation (Figure 6). To further characterize the B. infantis non-proteinaceous cytoplasmatic components, the possible involvement of bacterial genomic DNA fraction was tested. Addition to the organ bath of B. infantis DNA ranging from 0.5 μg to 10 μg did not modify the colon motility (data not shown).
The gut represents a complex and dynamic microbial ecosystem in which intestinal microflora has important and specific metabolic, trophic, and protective functions. Normal gut structure and function are the end-point of a complex set of interactions between the host and microorganisms colonizing the gut[2]. Several studies have shown that probiotics restore mucosal integrity and regulate the immune response. However, the effects of probiotics on the enteric nervous system and the intestinal musculature have not been systematically studied. This study was designed to investigate the motility response evoked by the probiotic preparation VSL#3 on the ileum and proximal colon isolated from guinea-pigs.
The choice of VSL#3, a formulation with a high viable concentration of Bifidobacterium, Lactobacillus and Streptococcus thermophilus, was based on the reported efficacy in bowel diseases associated with changes in propulsive motility of the gut, including diarrhea-predominant irritable bowel syndrome[18]. Furthermore, in clinical trials these exogenous probiotic bacteria have been shown to transiently colonize the human gut[19,21,22].
The data from this study show that the motility of isolated ileum is not influenced by the addition of either entire cells or cell debris of the VSL#3 mixture, whereas its crude extracts induce a dose-dependent contraction. It is noteworthy that VSL#3 crude extracts, which have a pH of about 5, and crude extracts in which the pH was increased up to physiologic value of 7.4 showed similar contractile responses. These results demonstrate that the ileum motility response is not related to the pH of the bacterial fraction and suggest that some bacterial metabolites could be involved in the contractions. Furthermore, as the presence of atropine (1 μmol/L) in the organ bath throughout the experiments did not determine a decrease of contraction induced by the addition of the VSL#3 crude extracts, the mechanism of action of the bacterial fractions does not involve muscarinic receptors.
Crude extracts of the different bacterial groups within the VSL#3 preparation provoke different motility response patterns. Bifidobacterium and Streptococcus strains did not change the basal tone of the ileum tissue, while Lactobacillus strains induced relevant ileum contractions. However, this contractile response is observed with volumes of cell extracts corresponding to lactobacilli concentration values of at least 107 CFU/mL, a value that is generally not detectable at ileum level of healthy humans[27]. This result suggests that the contractile effect evoked in ileum by VSL#3 cell extracts will be of minor importance in vivo at ileum level, as the rapid transit of ileum content and the low bacterial titer do not allow it to reach sufficient bacterial lysis or metabolic secretion. Furthermore, it is noteworthy that the contractile response induced by Lactobacillus is lower than that promoted by crude extracts of intestinal S. aboni added at the same concentration.
Like the guinea-pig ileum, the motility of guinea-pig proximal colon segment is modified by crude extracts of the VSL#3 mixture, while entire bacterial cells and cell debris do not exert any effect. The crude extract of all the probiotic strains (Bifidobacterium, Lactobacillus and S. thermophilus) included in VSL#3 induced dose-dependent relaxation of the proximal colon tissue, whereas the crude extracts of the control strains did not influence colon motility. These results suggest that the ability to induce a colon relaxation response is specific for these probiotic bacteria. Furthermore, the proximal colon relaxation response induced by probiotic bacteria cell extracts is reversible as successive washings abolished the colon relaxation. The restoration of the basal tone of the proximal colon tissue after removing the stimuli by several washings suggests that colonic tissue is not damaged. Volumes of cell extracts from Bifidobacterium, Lactobacillus and Streptococcus inducing the relaxation response correspond to bacterial titer values that are in the range of the physiological concentrations measured in colon[19].
Since B. infantis has been shown to colonize the human gut more efficiently than the other VSL#3 bacterial strains[22], we used this strain to further investigate the components of probiotic bacteria involved in the guinea-pig colon relaxation. Interestingly, only the cytoplasmatic fraction of the cell showed a pronounced colon relaxation activity. The bacterial cytoplasmic fraction may well have physiological implications, because its specific components reach the intestinal lumen as metabolites actively secreted by the viable bacteria colonizing the intestinal epithelium. In addition, the entire bacterial cytoplasm is released in the gut lumen following cell lysis. Further, subdivision of the bacterial cytoplasm allowed us to demonstrate that the non-proteinaceous cytoplasmatic portion was responsible for the colon relaxation, whereas protein factors had no effect on proximal colon relaxation. This result has been confirmed by challenging the colon tissue with bacterial cytoplasmatic fractions digested with proteinase K.
Since the cytoplasmatic fraction deprived of the protein component contains significant amounts of genomic DNA, which in prokaryotic microorganisms is not confined to a nucleus, the proximal colon segment was tested with the bacterial genomic DNA. The DNA preparation extracted from B. infantis did not cause any changes in colonic motility, indicating that other unidentified non-proteinaceous factors may be involved.
In conclusion, this is the first study performed in isolated tissues that deals with the effects of probiotic bacteria on intestinal motility. Our results suggest that Bifidobacterium, Lactobacillus and Streptococcus strains in VSL#3 mediate proximal relaxation activity. This could be one of the possible mechanisms of action by which probiotic bacteria exert their effects in ameliorating diarrhea by reducing stool frequency and restoring a disturbed microbial balance. Based on these data, further studies to elucidate the implication of a large array of receptor families and subtypes known to affect gut function and to characterize the non-proteinaceous bacterial molecules involved in intestinal motility are warranted.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 2. | Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2194] [Cited by in F6Publishing: 1995] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 3. | Marteau PR, de Vrese M, Cellier CJ, Schrezenmeir J. Protection from gastrointestinal diseases with the use of probiotics. Am J Clin Nutr. 2001;73:430S-436S. [PubMed] [Cited in This Article: ] |
| 4. | Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 422] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Saavedra J. Probiotics and infectious diarrhea. Am J Gastroenterol. 2000;95:S16-S18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33 Suppl 2:S17-S25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 263] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Chouraqui JP, Van Egroo LD, Fichot MC. Acidified milk formula supplemented with bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr. 2004;38:288-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Wunderlich PF, Braun L, Fumagalli I, D’Apuzzo V, Heim F, Karly M, Lodi R, Politta G, Vonbank F, Zeltner L. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J Int Med Res. 1989;17:333-338. [PubMed] [Cited in This Article: ] |
| 9. | McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90:439-448. [PubMed] [Cited in This Article: ] |
| 10. | Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163-169. [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 182] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | de dios Pozo-Olano J, Warram JH Jr, Gomez RG, Cavazos MG. Effect of a lactobacilli preparation on traveler’s diarrhea. A randomized, double blind clinical trial. Gastroenterology. 1978;74:829-830. [PubMed] [Cited in This Article: ] |
| 12. | Katelaris PH, Salam I, Farthing MJ. Lactobacilli to prevent traveler’s diarrhea. N Engl J Med. 1995;333:1360-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Hilton E, Kolakowski P, Singer C, Smith M. Efficacy of Lactobacillus GG as a Diarrheal Preventive in Travelers. J Travel Med. 1997;4:41-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Halpern GM, Prindiville T, Blankenburg M, Hsia T, Gershwin ME. Treatment of irritable bowel syndrome with Lacteol Fort: a randomized, double-blind, cross-over trial. Am J Gastroenterol. 1996;91:1579-1585. [PubMed] [Cited in This Article: ] |
| 15. | Verdu EF, Collins SM. Irritable bowel syndrome and probiotics: from rationale to clinical use. Curr Opin Gastroenterol. 2005;21:697-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Nobaek S, Johansson ML, Molin G, Ahrne S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 358] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol. 2001;152:735-741. [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 146] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, Zinsmeister AR. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 345] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 19. | Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 341] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 802] [Cited by in F6Publishing: 680] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 21. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1077] [Cited by in F6Publishing: 913] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 22. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 807] [Cited by in F6Publishing: 673] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 23. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114. [DOI] [Cited in This Article: ] [Cited by in Crossref: 660] [Cited by in F6Publishing: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 24. | Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol. 1999;65:351-354. [PubMed] [Cited in This Article: ] |
| 25. | Briejer MR, Bosmans JP, Van Daele P, Jurzak M, Heylen L, Leysen JE, Prins NH, Schuurkes JA. The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound. Eur J Pharmacol. 2001;423:71-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185380] [Cited by in F6Publishing: 187481] [Article Influence: 3471.9] [Reference Citation Analysis (0)] |
| 27. | Lammers KM, Brigidi P, Vitali B, Gionchetti P, Rizzello F, Caramelli E, Matteuzzi D, Campieri M. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 2003;38:165-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Chiarini A, Budriesi R, Bolognesi ML, Minarini A, Melchiorre C. In vitro characterization of tripitramine, a polymethylene tetraamine displaying high selectivity and affinity for muscarinic M2 receptors. Br J Pharmacol. 1995;114:1507-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Tallarida RJ, Murray RB. Manual of pharmacologic Calculations with Computer Programs. New York: Springer-Verlag 1987; . [Cited in This Article: ] |
| 30. | Motulsky H, Christopoulos A. Fitting Models to Biological Data Using Linear and Non Linear Regression. 2003; Available from: http://www.graphpad.com. [Cited in This Article: ] |
| 31. | Motulsky H. Statistic Guide: Statistical Analysis for Laboratory and Clinical Research: A Pratical Guide to Curve Fitting. San Diego (CA): GraphPad Software Inc.,. 2003; Available from: http://www.graphpad.com. [Cited in This Article: ] |