Published online Dec 7, 2008. doi: 10.3748/wjg.14.7009
Revised: November 7, 2008
Accepted: November 14, 2008
Published online: December 7, 2008
A 52 year-old male patient diagnosed of ankylosing spondylitis presented with an iron deficiency anemia after a ten-month treatment of methotrexate. He did not respond to treatment with oral iron not a proton pump inhibitor and an upper endoscopy was performed. The histological study of the duodenal biopsies showed villus atrophy. After removing the methotrexate, administrating intramuscular iron and undertaking a gluten-free diet, the histological and analytical alterations progressively resolved.
- Citation: Boscá MM, Añón R, Mayordomo E, Villagrasa R, Balza N, Amorós C, Corts JR, Benages A. Methotrexate induced sprue-like syndrome. World J Gastroenterol 2008; 14(45): 7009-7011
- URL: https://www.wjgnet.com/1007-9327/full/v14/i45/7009.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7009
Methotrexate is an immunosuppressive agent commonly used in the daily practice of many specialties. Methotrexate, in a weekly dose, can be used for years where its use is primarily limited by toxicity. The main side effects include renal or liver impairment, orointestinal mucositis, bone marrow toxicity and gastrointestinal side effects, such as nausea or vomiting, many of which can often be avoided by using low-doses of the drug, or combining it with folic acid supplementation[1].
Several cases of villus atrophy, after the use of immunosuppressors[2], have been reported in the medical literature; however, only one case of intestinal villus atrophy secondary to Methotrexate, has been described[3]. We present a second case of a sprue-like syndrome secondary to methotrexate treatment, this time in a paucisymptomatic patient.
A 52 year-old male patient was referred to our Gastroenterology out-patient clinic from the Rheumatology Department, as he presented with a progressively increasing iron deficiency anemia, that did not respond to proton pump inhibitors and iron taken orally.
He had been diagnosed of ankylosing spondylitis, HLA B-27 positive, in 2000, and was initially treated with the nonsteroidal antiinflammatory drugs (NSAIDs), salazopyrine and omeprazol. In April, 2002, treatment with salazopyrine was stopped, but prednisolone and methotrexate were added in October, 2002, because the patient had severe arthralgias. The arthralgias lessened with the new treatment, but analytical alterations were progresively seen. An analysis during December, 2002, showed normal hemoglobin (Hb 13.4 g/dL, VCM 87 and HCM 30.2) and iron (70 μg/dL), but in January, 2003, the hemoglobin level had decreased to 11.7 g/dL. By May, 2003, the asymptomatic patient presented with iron deficiency anemia (Hb 9.9 g/dL, Hto. 31%, VCM 71, HCM 22.9, platelets 489000, iron 22 μg/dL, transferrine 366, IST 5% and ferritine 2 ng/dL), for which he received iron, taken orally, and was referred to the Gastroenterology Department.
He had no digestive symptoms, weight loss, or anorexia. The suspected diagnosis included: (1) gastric erosions secondary to NSAIDs, (2) a side-effect of Methotrexate, or (3) other causes of iron deficiency anemia. Therefore, an endoscopy and celiac sprue antibodies were requested, and treatment with oral iron and proton pump inhibitors continued.
The upper endoscopy showed a stomach with patched mucosa, alternating red and white areas, which continued in the first and second portions of the duodenum, in which many longitudinal erosions, covered with fibrin, were observed, macroscopically compatible with an Inflammatory Bowel Disease, or a Lymphoma.
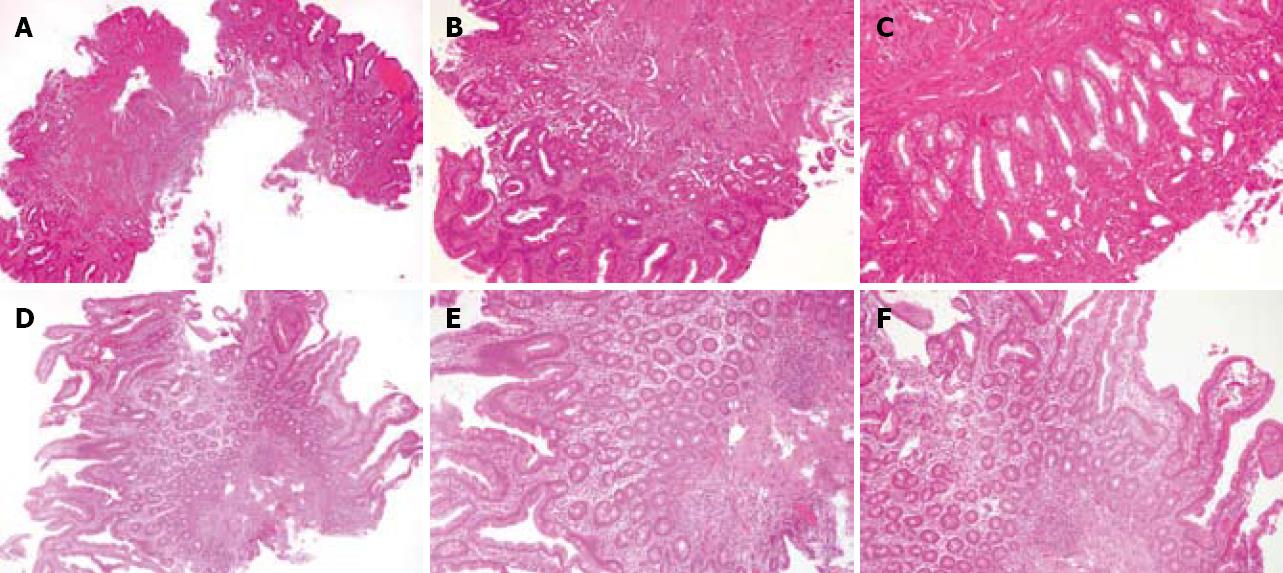
However, the histological study showed intestinal biopsy with evidence of atrophy of the wall, with decreased thickness, increased collagenous fibers in the interstitium, mucosal flattening of the villi and small repare glands (Figure 1A). The mucosa shows a loss of glandular structure, with small and round reparing glands, covered by a cubical one-layer epithelium, with nuclei containing reactive atypia, with mild pleomorphism, larger in size and variable nucleoli. There was a fibrous stroma and a heterogeneous inflammatory infiltrate, with eosinophil leukocytes in moderate quantity (Figure 1B-C).
In July, since the hemogram was similar (Hb 9.2 g/dL), despite three months of oral iron, antibodies were negative, and taking into account the results of the upper endoscopy, an intestinal follow-through and a colonoscopy (which were both normal) were requested and methotrexate was discontinued.
Three months later, the iron deficiency anemia persisted and a gluten-free diet was tested. In January, 2004, after three months of a gluten-free diet and six months of not taking methotrexate, there was still a iron deficiency anemia with a hemoglobin of 9.4 g/dL, a new endoscopy was performed. The upper endoscopy showed a loss of duodenal folds and the urease test for Helicobacter pylori was negative. The histological study confirmed the existence of an atrophic gastritis and duodenitis. With the result of the endoscopy, the patient returned to our out-patient-clinic in March, 2004. He had an itchy eruption, made up of clusters of tiny blisters in the elbows and back, which was compatible with a Dermatitis Herpetiformis. He was referred to the Dermatology out-patient clinic, but was not visited until six weeks later where the skin lesions had spontaneously disappeared.
Treatment with intramuscular iron was initiated after the Dermatologic evaluation in April, 2004, and three months later the hemogram was almost normal; there was no anemia, (Hb 13.1 g/dL), but there was still a slight hypocromia and microcytosis. The intramuscular iron was stopped in December, 2004, when the still asymptomatic patient reached acceptable levels of iron, ferritine and transferrine (Fe 158 μg/dL, Transf. 275 mg/dL and FE 93 ng/mL) and had a normal hemogram (Hb 14.5 g/dL, Hto. 43%, VCM 84, HCM 28.5).
The patient was periodically seen in our out-patient clinic the following year. Analysis remained normal and a control upper endoscopy was carried out on December, 2005. It showed a dramatic change from the first one, which had been performed two years earlier, when the patient was undergoing treatment with methotrexate. The endoscopy was macroscopically normal.
The histological study showed an intestinal biopsy with normal wall thickness, normal size and number of villi, as well as normal gland and stromal density (Figure 1D). Many intestinal glands, covered by a one-layer cylindrical epithelium with goblet cells, were observed. Between them, the interstitium showed very mild inflammation with a decrease in the fibrous weft. Inflammatory cells were heterogeneously composed of lymphocytes and plasmatic cells (Figure 1E-F).
The patient remains asymptomatic, from a gastrointestinal point of view, and without anemia or ferropenia. We have not rechallenged methotrexate for ethical reasons.
The interest of this case is the appearance of a sprue-like syndrome after the use of methotrexate, and its complete resolution, following the removal of the drug. Although several cases of small-bowel villus atrophy have been described with other immunosuppresive agents, like Azatioprine[2], to our knowledge, only one similar case has been described regarding methotrexate[3]. The latter presented a case of diarrhea, progressive weight loss and general malaise after two years of low-dose methotrexate. Biopsies taken during the treatment showed small-bowel villus atrophy and confirmation of mucosal healing was carried out months after removal of methotrexate.
Our patient did not display any symptoms. He only had an iron deficiency anemia. Six months after beginning low-dose methotrexate (15 mg/wk), a iron deficiency anemia developed. He showed no signs or symptoms of mucositis, or symptoms of bone marrow toxicity, or of renal or liver impairment. No diarrhea, nausea or other gastro-intestinal symptoms were present. We believe he had an iron malabsorption, secondary to duodenal atrophy, which slowly resolved after removing methotrexate, with clinical, analytical, endoscopical and microscopical confirming the healing.
This drug is frequently used in patients with rheumatological, gastroenterological and oncological illnesses. At high doses, and without folic acid supplement-ation, mucositis is a side-effect in which experimental studies have tried to understand[4,5] for prevention[6-7]; however, not many human cases have been described. Its presentation is usually symptomatic (weight-loss, diarrhea, nausea, general malaise, etc) and tends to appear with high doses.
The pathogenesis of the sprue-like syndrome is unclear. Two mechanisms might be involved, local antimetabolite toxicity and genetic predisposition[3].
Our case might allow other clinicians to think of an underlying sprue-like syndrome when a iron deficiency anemia appears when taking methotrexate, even if, like in our case, the patient is taking low doses of the drug and is completely asymptomatic.
This methotrexate-induced sprue-like syndrome is of clinical interest because of its singularity in the clinical presentation (only iron deficiency anemia), its origin (a duodenal atrophy induced by low-dose methotrexate, with no myelosuppression), and its complete resolution after withdrawal of the drug, which has been confirmed both through the periodical analysis and through the endoscopical study.
Peer reviewers: Dr. Wang-Xue Chen, Institute for Biological Sciences, National Research Concil Canada, 100 Sussex Drive, Room 3100, Ottawa, Ontario K1A 0R6, Canada; Hallgrimur Gudjonsson, MD, Gastroenterology, University Hospital, University Hospital, Landspitali, Hringbraut, Reykjavik 101, Iceland; Daniel C Baumgart, MD, PhD, FEBG, Division of Hepatology and Gastroenterology, Department of Medicine, Charité Medical School, Humboldt-University of Berlin, Virchow Hospital, Berlin D-13344, Germany.
S- Editor Li JL L- Editor Rippe RA E- Editor Lin YP
| 1. | Hoekstra M, van Ede AE, Haagsma CJ, van de Laar MA, Huizinga TW, Kruijsen MW, Laan RF. Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:423-426. [Cited in This Article: ] |
| 2. | Ziegler TR, Fernandez-Estivariz C, Gu LH, Fried MW, Leader LM. Severe villus atrophy and chronic malabsorption induced by azathioprine. Gastroenterology. 2003;124:1950-1957. [Cited in This Article: ] |
| 3. | Houtman PM, Hofstra SS, Spoelstra P. Non-coeliac sprue possibly related to methotrexate in a rheumatoid arthritis patient. Neth J Med. 1995;47:113-116. [Cited in This Article: ] |
| 4. | Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180-188. [Cited in This Article: ] |
| 5. | Carneiro-Filho BA, Lima IP, Araujo DH, Cavalcante MC, Carvalho GH, Brito GA, Lima V, Monteiro SM, Santos FN, Ribeiro RA. Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig Dis Sci. 2004;49:65-72. [Cited in This Article: ] |
| 6. | Harsha WT, Kalandarova E, McNutt P, Irwin R, Noel J. Nutritional supplementation with transforming growth factor-beta, glutamine, and short chain fatty acids minimizes methotrexate-induced injury. J Pediatr Gastroenterol Nutr. 2006;42:53-58. [Cited in This Article: ] |
| 7. | Yuncu M, Eralp A, Koruk M, Sari I, Baqci C, Inaloz S. Effect of vitamin A against methotrexate-induced damage to the small intestine in rats. Med Princ Pract. 2004;13:346-352. [Cited in This Article: ] |