JOURNAL OF CARDIOLOGY AND CARDIOVASCULAR RESEARCH
Re-analysis of Coronary Intravascular Lithotripsy - Can It Be Used in All Comers?
Rohit Mody1*, Debabrata Dash2, Bhavya Mody3, Anand Reddy Maligireddy4, Ankit Agarwal5, and Lakshay Rastogi6
1Department of Cardiology, MD, DM, MAX Super specialty hospital, Bathinda, Punjab, India
2Department of Cardiology, MD, DM, FICC, FAPSC, FCCP, FSCAI, Aster Hospital, Dubai, Al Quasis, UAE
3Department of Medicine, MBBS, Kasturba Medical College, Manipal, Karnataka, India
4Department of Cardiology, Mayo Clinic, 13400 E Shea Blvd, Scottsdale, AZ 85259, USA
5Department of Cardiology, Cleveland Clinic, 9500 Euclid Avenue Cleveland, Ohio 44195, USA
6Department of Cardiology, Kasturba Medical College, Manipal, India
*Corresponding Author: Rohit Mody, Department of Cardiology, MD, DM, MAX Super specialty hospital, Bathinda, Punjab, India.
| ReceivedApr 4, 2022 | RevisedApr 18, 2022 | AcceptedApr 22, 2022 | PublishedApr 28, 2022 |
Abstract
In severely calcified lesions, the intravascular lithotripsy (IVL) can be an effective therapy for preparation of the lesion so that drug eluting stent (DES) can be deployed optimally, and the future events of stent failure can be minimized. From the recent studies with IVL we can certainly say it is a novel therapy. The mechanism as depicted by optical coherence tomography (OCT) is found to be calcium fractures induced by shockwaves. The above said modification is done with minimal complications of perforation, no reflow, low incident of flow limiting dissection and myocardial infarctions. The other methods like cutting or scoring balloons, rotational atherectomy have also shown to improve luminal diameter, but other complications like distal embolization is a challenge of concern. This review reports a single centre study to all comer patients even with complex characteristics. This therapy was found to be very effective and with minimal complications. In this article we describe the mechanism of action, about IVL catheter, its OCT validation, clinical uses, its comparison with other calcium modifying technologies and what other future directions can be utilized to improve the technology and can it be used in all comers with calcified coronaries.
Keywords
Intravascular lithotripsy; Optical coherence tomography; Micro-catheter; Corsair; Tornus; Penetrability
Abbreviation
ACS: Acute Coronary Syndrome; ATM: Ataxia Telangiectasia Mutated; CFA: Common femoral artery; CTO: Chronic Total Occlusion; DAPT: nDual Antiplatelet Therapy; DES: Drug-Eluting Stent; EHL: Electrohydraulic Lithotripsy; ESWL: Extracorporeal Shockwave Lithotripsy; PAD: Peripheral Arterial Disease; PCI: Percutaneous Coronary Intervention; RVD: Reference Vessel Diameter; TVR: Target Vessel Revascularization.
What is known?
- Stent clotting can be prevented by treating calcified coronary lesions after optimal plaque preparation.
- Percutaneous coronary interventions including high and ultra-high-pressure dilatation, scoring/cutting balloons, and atherectomy have all been related to an increased risk of future events.
- When preparing lesions in coronary arteries that are heavily calcified, intravascular lithotripsy is an innovative new method to use.
- Patients with heavily calcified coronary lesions, lesions resistant to high-pressure balloon dilatation (secondary intravascular lithotripsy), and patients who had previously deployed under expanded stents had intravascular lithotripsy.
- An appropriate method for preparing coronary lesions with calcified lesions, intravascular lithotripsy has an acceptable success rate, minimal complications, and low risks of major cardiovascular events.
Future directions
- There are various areas where IVL catheter system can be iterated and improved in deliverability, size, and number of pulses.
- Long term outcome studies are required.
- All comers need study. All comers like vein grafts, ostial lesions, ACS, In-stent restenosis need study.
- IVL and other calcium-modifying treatments must be compared in randomized clinical trials.
Introduction
A decrease in arterial compliance and an increase in the risk of complications following revascularization are both brought on by vascular calcification that occurs after the PCI [1-3]. Device crossing and stent apposition and expansion are hindered by coronary artery calcification [4,5], and drug-eluting polymer delamination [6,7], as well as drug delivery and elution kinetics are changed. All of these effects have a negative impact on the outcomes of coronary treatments [8]. Underexpansion of the stent is related to stent thrombosis and/or the requirement for lesion revascularization [9] Noncompliant balloons and atheroablative procedures for decreasing coronary artery calcification have limitations that must be addressed while using these therapies. Noncompliant or customised balloon dilatation may not be forceful enough to shatter calcium and widen the artery, resulting in barotrauma-related dissection or perforation of the artery. An eccentric ''rut or trough'' might be formed by the wire bias during rotational or orbital atherectomy, with no influence on the calcium's circumference [10]. When compared to a balloon alone, atheroablative technology may cause slow flow, myocardial infarction, flow-limiting dissection, distal embolization, and perforation [7,11,12]. Vascular calcification may now be treated using a new procedure known as intravascular lithotripsy (IVL). Renal calculi are addressed using acoustic pressure waves, however, the IVL approach has unique delivery changes to target vascular calcium. It is possible to deliver localised pulses of sound pressure across the arterial circumference using an adaptable balloon angioplasty catheter with lithotripsy emitters (acoustic pressure wave sources) attached to the shaft of the balloon [13,14]. In patients with highly calcified coronary and peripheral artery disease, IVL's safety and effectiveness have been shown in multiple clinical trials [15,16].
IVL acoustic pressure waveform modification
Electric sparks from IVL emitters cause vapour bubbles to form within a balloon's fluid medium, which is then trapped inside the balloon. Vessel walls are subjected to circumferential acoustic pressure waves created by IVL because of the low quantity of electric energy generated by the device. It is possible to alter the pulse width communicated to the emitters to create a smaller negative peak pressure (about 3 atm) and a lower positive peak pressure (roughly 5 MPa) than the ESWL. ESWL and IVL shockwaves, like IVL, move through soft tissues with minimal impact. When IVL comes into contact with calcium, one of the primary mechanisms for calcium fracture is compression stress. IVL shockwaves have a low peak negative pressure and tensile stress, unlike ESWL shockwaves, which do not cause tissue damage (about 0.3 MPa or 3 atm). As a result, the amount of tissue damage that may occur is minimized [17]. IVL shock waves have a lower acoustic energy flow density owing to their unfocused nature (the acoustic energy per unit area). To fracture calcium rather than Pulver it, IVL uses far less energy than ESWL since the IVL shockwaves are sent directly to the target calcium in the vessel. The IVL energy flow density is sufficient to fracture calcium in the artery while reducing the risk of soft tissue injury caused by higher energy.
IVL catheter sizing and emitter alignment
The relevance of IVL balloon size and emitter alignment was emphasized in a post-hoc assessment of the Disrupt PAD II (Shockwave Lithoplasty DISRUPT Experiment for PAD) experiment. In the superficial femoral and popliteal arterial segments, IVL alone resulted in an optimal 12-month primary patency rate [18]. Correct balloon size and therapeutic error avoidance were part of the best procedure definition. The ratio of the IVL blimp to the reference vessel diameter (RVD) was used to ascertain balloon sizing, with a ratio of ≥1 being positive and fulfilling. As an instance of a therapeutic miss, failure to perform complete lithotripsy on an overlapping section or at the lesion location was described. The 12-month primary patency was significantly improved, and clinically induced target lesion revascularization was reduced, when the optimal IVL method was used. Importantly, there was no significant increase in adverse outcomes when the IVL balloon size passed the RVD. IVL recommendations suggest using a 1:1 IVL catheter size for treating long lesions. The manufacturer's instructions for using the Shockwave C2 recommend a 1:1 IVL balloon-to-target vessel RVD ratio (currently approved to be used outside the United States).
Optical coherence tomographic visualization of IVL-induced calcium fracture
Calcium fracture going to follow IVL treatment was comparable in micro-computed tomographic and histopathologic findings from cadaveric studies in the Disrupt PAD II OCT sub analyses in the Disrupt CAD I (Shockwave Coronary Rx Lithoplasty® Study), Disrupt CAD II (Shockwave Coronary Lithoplasty® Study), and Disrupt CAD III (Disrupt CAD III With the Shockwave Coronary IVL System) studies [21].
A number of OCT sub studies have found the following:Vascular gain following IVL treatment can be attributed to calcium fracture rather than high-pressure balloon dilation; IVL causes circumferential and longitudinal fracture of calcium; and IVL treatment promotes vascular compliance and enables for the expansion of stents in coronary vessels to expand. When the balloon is expanded to the same pressure in this clinical setting, the artery luminal area increases. Because advanced coronary disease reduces both intima and medial thickness, OCT imaging can be difficult to distinguish between these two types of calcium deposits in coronary vessels. The coronary arteries do not show medial calcification, which is frequent in peripheral arterial disease. As a result, the labels "superficial'' and ''deep'' for calcium distribution may be more appropriate for coronary calcification. Calcified coronary and peripheral arteries can be treated with IVL without respect to anatomical differences [16,19,23,24]. IVL treatment's effect on eccentric and nodular calcium has not been studied thoroughly enough. Moreover, since patients with peripheral artery disease are more likely to use intravascular ultrasonography, OCT imaging of IVL treatment in peripheral arteries is limited. Treatment with fluid resuscitation. The angiographic data of 336 patients who had treatment with the Shockwave Lithoplasty® System and the Disrupt PAD I, PAD II, and PAD III investigations were analyzed, and there was no difference in the ultimate diameter stenosis in 336 of the 336 patients who had treatment with the Shockwave Lithoplasty® System and the Disrupt PAD I, PAD II, and PAD III investigations. Excentric and concentric lesions were shown to have similar angiographic outcomes, as were their consequences, in Disrupt CAD I and II [25] patient-level pooled angiographic data [25]. An OCT substudy found that stent expansion (>100%) was almost identical in the region of greatest calcium thickness, irrespective of the angle of the calcium. In particular [20]. A further analysis will look at the influence of IVL therapy on the pattern of eccentric and nodular calcium using data from the Disrupt CAD I, II, III, and IV OCT substudies (n=250).
As a result of IVL-mediated calcium reduction, there was an increase in vascular minimum luminal area in both peripheral and coronary arteries following low-pressure balloon treatment [19-21]. Imaging using OCT revealed both circumferential and longitudinal calcium fractures in several directions, with the fracture breadth growing after the stent expansion of IVL treatment [19]. These data suggest that IVL enhances vascular compliance and allows for optimum stent expansion through calcium fracture. IVL treatment has been shown to cause deep calcium fractures in the adventitia, however no arterial perforations were found in the Disrupt CAD or Disrupt PAD studies with IVL alone. This indicates that IVL can modify deep vascular calcium [18-20,26,27]. IVL shockwave damage and adventitial fibrosis, which have been documented in advanced coronary and peripheral artery disease, are likely to reduce arterial perforation [28-31]. 67.7 percent of the lesions treated with disrupt CAD III after IVL showed signs of calcium fracture. Using OCT imaging to evaluate lesions with and without apparent calcium fracture, there were no differences in the minimal stent, luminal, or stent expansion areas in the location of the greatest calcification [19]. Because of ''out of plane'' fractures or microfractures that are too small to be seen by current OCT technology, even while OCT imaging appears to show a calcium fracture, a discrepancy could exist between that and the ideal stent insertion indices [32,33].
Clinical use of IVL
Learning curve assessment
IVL technology uses the same angioplasty balloon catheters that are used for coronary and peripheral intervention to create a safe, effective, and intuitive (operator-friendly) technique to treat hard calcified lesions. In Disrupt CAD III, the first coronary IVL experience for American interventional cardiologists, there were no significant differences in procedural success, device crossing success, or 30-day freedom from major adverse cardiovascular events between the 47 roll-in procedures (the first case at each international site) and the subsequent 384 intention-to-treat procedures.
IVL in clinical practice
The Shockwave M5 and S4 IVL catheters may be used to treat peripheral vascular disease in Europe, the United States, and other countries. Clinical research have proven that a variety of peripheral vascular beds are both useful and safe. Regardless of the vascular bed, calcium severity, calcium distribution (eccentric or concentric), or patient risk subgroup, all 336 patients with moderate to highly calcified peripheral lesions received IVL treatment [23]. Because to IVL facilitation, large-bore catheter placement through transfemoral artery access for transcatheter aortic valve replacement, endovascular abdominal or thoracic-aortic stent grafts, and mechanical cardiac support devices (such as Impella) are acquiring more clinical expertise [34-36]. ''Converting'' to transfemoral access is possible for many patients previously thought unsuitable for this method of access, thereby eliminating the hazards of general anesthesia, hospitalization, and higher procedure-related resources. A ''bail-out'' stent may be necessary following an IVL procedure because IVL perforation, difficult dissection, and the need for a ''bail-out'' are uncommon.
The Shockwave C2 IVL catheter has been commercially marketed in Europe since the end of Disrupt CAD I in 2018 [24]. Pre-market trials for US-based Disrupt CAD III and Japanese research on Japanese Disrupt CAD IV were completed in 2020 for all participants. [19,37]. In all of the Disrupt CAD investigations, patients were treated who had calcified target lesions and stable ischemic heart disease (IHD). Disrupt CAD's safety and effectiveness were consistently high across all of its clinical studies. Each of the Disrupt CAD investigations had OCT substudies that were assessed by an independent core laboratory and showed numerous planes of calcium fracture, proving that calcium fracture is the key mechanism of action for improved vascular compliance with IVL therapy [16,20]. The application of IVL in a variety of anatomic and procedural situations across a wide range of target lesion calcium morphologies has led to an increase in clinical expertise in coronary IVL (Supplemental Table 1). In spite of the fact that the most effective percutaneous coronary intervention strategy for calcified lesions is still being developed, the Society for Cardiovascular Angiography and Interventions recently released an algorithm that incorporates intravascular imaging and calcium-modifying technologies like IVL to guide treatment of these difficult coronary lesions [38].
Comparison with other calcium modifying technologies
There are several reasons why IVL is better to balloon-based technologies (such as cutting/scoring balloons) in the treatment of calcified lesions (rotational or orbital atherectomy). While balloons are commonly used to shift plaques, IVL use a semi-compliant balloon that is inflated to roughly 4 atmospheres, which avoids the high-pressure inflation and the risk of barotrauma associated with noncompliant balloons. As a starting point, atheroablative technologies are based on the removal of surface calcium, which results in thermal damage to the arteries that are targeted. Eccentric ruts or troughs can emerge as a result of the wire bias in their action, which raises the possibility of incomplete calcium modification.
The inability of atheroablative technologies to alter deep calcium, which might compromise procedure safety, is compounded by the absence of an appropriate wire bias or larger device sizes. When calcium is broken in situ via IVL, the risk of vascular issues and thermal injury is reduced. Table 1 shows that calcium-modifying technologies can produce vascular issues at varying rates in target lesions with substantial calcium.
IVL | Rotational Atherectomy | Orbital Atherectomy | Laser Atherectomy | |
| Study | Disrupt CAD I, Disrupt CAD II, Disrupt CAD III,
Disrupt CAD IV [16,19,24,37] | Prepare-CALC [39] | ORBIT II [40] | Bilodeau et al. [41] |
| n | 60, 120, 384, 64 | 100 | 443 | 95 |
| Moderate to severe Caþþ, % | 94.2–100 | 100 | 100* | 80%† |
| Angiography core laboratory | Yes | Yes | Yes | Yes |
| In-hospital MI, % | 5.0–6.8‡ | 2.0§ | 9.3‡ | 2.1 |
| Dissection (types D–F), % | 0.0–0.3 | 3.0|| | 0.9¶ | 5.3¶ |
| Perforation, % | 0.0–0.3 | 4 | 0.9 | 0 |
| Abrupt closure, % | 0.3 | NR | 0.2 | 0 |
| Slow flow, % | 0 | 2.0# | 0.5 | 0 |
| No reflow, % | 0 | - | 0 | - |
Table 1: Angiographic Complications with Coronary Calcium Modification Technologies.
Future directions
There are several areas where the IVL catheter system might be iterated and improved, hence enhancing the technology's clinical applicability. First and foremost, the IVL catheter's lower crossing profile and improved flexibility may aid delivery in stenotic or convoluted lesions. Because the EHL emitters are included into the IVL catheter's shaft, the current-crossing profile is bigger with IVL catheters. Delivery is influenced by cross-profile and flexibility. Longer IVL catheter shaft lengths and larger IVL balloon sizes can also be used to treat more distant lesions, which can be used in both coronary (left main) and peripheral vessels. Since the maximum balloon matrix for Shockwave IVL catheters (4.0mm) and IVL catheters for peripheral arteries (S4 (4.0mm) and M5 (7.0mm) may be expanded, this will provide a better match between the size of the IVL balloon and the artery and greater transmission of sound energy. Increased pulses per IVL catheter may also allow more patients to be treated with a single catheter, thereby lowering the procedure's length and expense.
We need to monitor these patients for a longer period of time in order to determine the long-term effects of IVL's acute findings, such as optimal stent expansion and limited stent area. It will be possible to examine the effectiveness of intracoronary IVL-assisted stent implantation in patients who participated in Disrupt CAD III throughout the course of a two-year follow-up period. Patients with unstable coronary syndromes, in-stent restenosis or under expanded coronary stents [34-37,42] significant calcification in upper extremity vessels (i.e., carotid arteries and vein grafts, subclavian/axillary arteries and vein grafts, and infrarenal aorta, radial, and brachial) and vein grafts.
Following FDA approval of the Shockwave Coronary IVL System, an investigator-sponsored ''all-comers'' registry (REPLICA [Registry of Coronary Lithotripsy in Spain; NCT04298307],N=400) is now enrolling in Spain, and a post-market approval study using the American College of Cardiology National Cardiovascular Data Registry CathPCI Registry is being developed to provide insights into device safety and effectiveness in a wider ''real-world'' setting. Additional randomized controlled pilot studies comparing IVL to cutting or scoring balloons (BALI[Balloon Lithoplasty for Preparation of Severely Calcified Coronary Lesions;NCT04253171], N=200; CCS [Coronary Calcification Study; NCT04428177], N=40) or rotational atherectomy and laser atherectomy (ROLLERCOASTER[Rotational Atherectomy, Lithotripsy, or Laser for the Treatment of Calcified Stenosis; NCT04181268], N=150) are planned to evaluate the safety and effectiveness of IVL compared with other calcium-modifying technologies in severely calcified coronary lesions prior to stent implantation.
Disrupt CAD I, II, and III, OCT sub studies are now being conducted to address these concerns, since evidence of IVL therapy's effectiveness in treating eccentric and/or nodular calcification is also lacking. In order to guide the treatment of calcified lesions in coronary and peripheral arteries, a randomized clinical study evaluating the safety and efficacy of IVL and other calcium-modifying medications is required (e.g., high-pressure balloon, atheroablative technologies, laser atherectomy). Aortic valve calcifications, mitral regurgitation, and the mitral annulus are all areas of potential investigation using IVL.
What did we study?
Shockwave IVL catheter (Translumina) followed by DES implant has been used to treat 45 patients with calcified lesions between July 2021 and December 2021. They were further divided into 9 categories:
1. Primary IVL group with Calcified lesions-10 lesions
2. IVL in calcified nodules-4 lesions
3. IVL in calcified LM bifurcations-3 Bifurcation lesions [6]
4. IVL in under deployed stents-6 Lesions
5. IVL in ISR – Calcified-3 lesions
6. IVL in large vessels & eccentric Calcium-5 lesions
7. IVL in CTO PCI-2 lesions
8. IVL in uncrossable lesions-4 lesions
9. IVL in Long Lesions-5 lesions
After PCI these patients were studied angiographically and with imaging with IVUS and the outcomes were recorded, the clinical events like cardiac death, TVR & MI were noted for 1 month follow-up.
How was the study executed?
IVT is examined in the context of IVL patients treated at a single data center. Shockwave balloon-based coronary catheter system was used for IVL on all patients in order to conduct a clinical audit, which involved reviewing medical records, anomysing data, and conducting IVL on all patients (Translumina). The balloon catheter was inflated to 4 atm at the affected site and a 0.014' coronary wire was used to deliver up to ten pulses per second for ten seconds. After the pulses were administered, the balloon was inflating to 6 atm. After that, you may get up to 80 additional impulses.
In situations where there were many lesions present, a minimum of 20 pulses were used to treat each one.Patients undergoing PCI-PCI underwent the procedure in accordance with traditional and local standards, both at the same location and in a staged manner. DAPT was given to all patients, and intra-arterial heparin was administered to keep them safe during the treatment.
What are the essential results?
In 45 cases, the target lesion was successfully treated with IVL (100 percent). After delivering 15 and 30 pulses, the IVL balloons burst in seven occasions in the major IVL subgroup. It didn't, however, cause any problems. 7 lesions had minor dissections (type A to C dissections) (15 percent). Coronary perforation was not seen in any of the individuals". Neither an abrupt vessel closure nor even a delay in the flow of blood or a reflow occurred during this study. Angiographic and clinical success were attained in 100% and 96% of the cases, respectively, of the 45 lesions that were treated.
It was shown that just two patients (4 percent) had MACE secondary to in-hospital MACE (which included two TVR). Three MACEs occurred in two individuals at the 30-day follow-up period (4 percent). At 30 days, there were no MACEs in the major IVL subgroup(Figure 1).
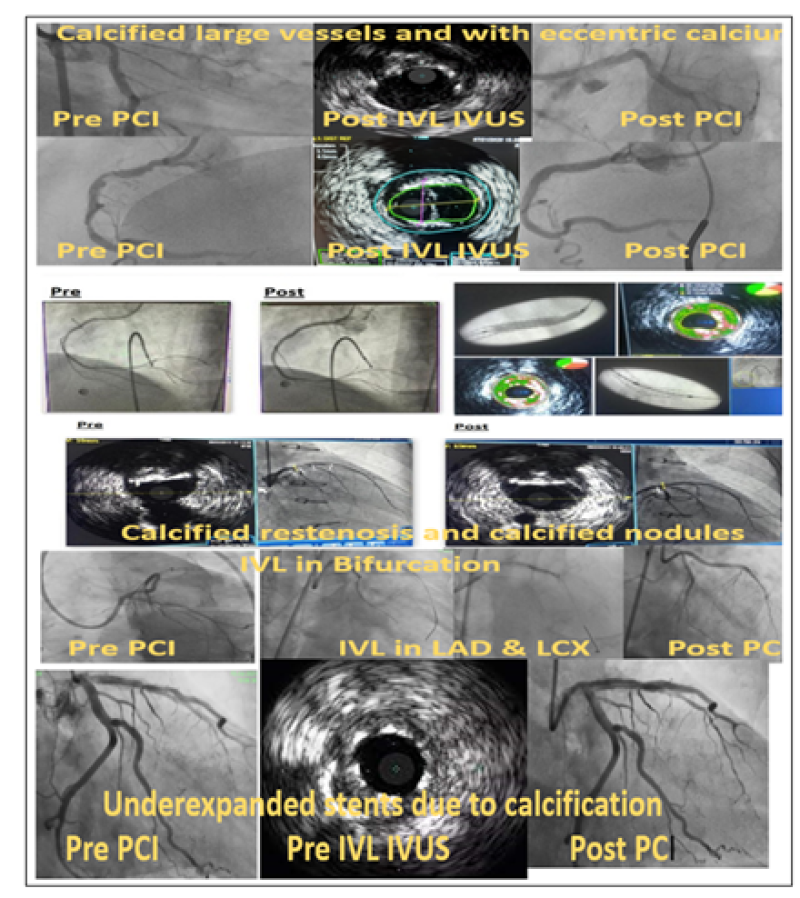
Figure 1: IVL in various PCI subsets.
Why is this important?
In the treatment of coronary artery plaques with a high level of calcium, intravascular lithotripsy is an important innovation. During the procedure, sonic pressure waves generated by vaporization fluid are used to interact with artery calcification. A substantial body of research shows that IVL increases vascular compliance prior to stent placement with great efficacy and minimal risk. Because of increasing operator experience and the CE mark, IVL is now being used in more challenging clinical situations.This retrospective observational data in real world shows that after IVL we see fractures of calcium in 60% of our cases and there is definite acute lumen gain. Inpatients in which we cannot realize fractured calcium by imaging still we get good expansion of deployed stents.
In our study it is clear that IVL can be used across all types of calcium subsets which we define on Imaging also across complex scenarios like LM Bifurcation and CTO.
Also lesions which are uncrossable can be pre-dilated with CTO balloons and the IVL balloon can be crossed with maneuvers like Anchor Balloon Technique, use of Buddy wire & use of guideliner support.
In nutshell this technique with IVL is useful across wide range of morphologies, calcium subtype and even in stent failure.
The essentials to remember
Why?
All patients who had severely calcified coronaries on Angiography and IVUS imaging, The plaque modification with IVL is important to deploy a stent which is fully expanded and is expected to translate into good long-term outcomes.
What?
All subsets of patients with complexity like CTO & LM also cases of stent failure especially under-deployed stents and all type of morphologies of calcium including calcified nodules and eccentric calcium are studied.
How?
With 1:1 ratio of IVL catheter balloon, IVL pulses are given which modify the calcified plaque and procedure is completed by deploying DES.
What are the results?
Primary endpoint- Angiographic results 100% success, clinical success was 96%. Secondary endpoint- MACE occurred in 2 Patients (4%), no MI & deaths were noted.
Why is this important?
From this small observational study, IVL is hypothesized to be effective against all type of calcified lesions the acute luminal gain and good expansion of stent seen by imaging may translate into good long-term clinical outcomes.
References
1. RochaSingh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinicalimplications. Catheter Cardiovasc Interv. 2014;83(6):212-20. PubMed | CrossRef
2. Mattesini A, Di Mario C. Calcium: A predictor of interventional treatment failure across all fields of cardiovascular medicine. Int J Cardiol. 2017;231:97-8. PubMed | CrossRef
3. Lee MS, Shah N. The impact and pathophysiologic consequences of coronary artery calcium deposition in percutaneous coronary interventions. J Invasive Cardiol. 2015;28(4). PubMed
4. Mori S, Yasuda S, Kataoka Y, Morii I, Kawamura A, Miyazaki S. Significant association of coronary artery calcification in stent delivery route with restenosis after sirolimus-eluting stent implantation. Circ J. 2009;73(10):1856-63. PubMed | CrossRef
5. Kini AS, Vengrenyuk Y, Pena J, Motoyama S, Feig JE, Meelu OA, et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. 2015;86(6):1024-32. PubMed | CrossRef
6. Wiemer M, Butz T, Schmidt W, Schmitz KP, Horstkotte D, Langer C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc Interv. 2010;75(6):905-11. PubMed | CrossRef
7. Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63(17):1703-14. PubMed | CrossRef
8. Tzafriri AR, Garcia-Polite F, Zani B, Stanley J, Muraj B, Knutson J, et al. Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis. J Control Release. 2017;264:203-10. PubMed | CrossRef
9. Généreux, P, Madhavan MV, Mintz GS, MaeharaA, Palmerini T, LaSalle L, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes: pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trials. J Am Coll Cardiol. 2014;63(18):1845-1854. PubMed | CrossRef
10. Yamamoto MH, Maehara A, Karimi Galougahi K, Mintz GS, Parviz Y, Kim SS, et al. Mechanisms of orbital versus rotational atherectomy plaque modification in severely calcified lesions assessed by optical coherence tomography. JACC Cardiovasc Interv. 2017;10(24):2584-6. PubMed | CrossRef
11. Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist V, Meinertz T, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6(1):10-19. PubMed | CrossRef
12. Matsuo H, Watanabe S, Watanabe T, Warita S, Kojima T, Hirose T, et al. Prevention of no-reflow/slow-flow phenomenon during rotational atherectomy-a prospective randomized study comparing intracoronary continuous infusion of verapamil and nicorandil. Am Heart J. 2007;154(5):994. PubMed | CrossRef
13. Powers CJ, Tinterow MM, Burpee JF. Extracorporeal shock wave lithotripsy: a study of renal stone differences. Kans Med. 1989;90(1):19-22. PubMed | CrossRef
14. Cleveland RO, McAteer JA. Physics of shockwave lithotripsy. In: Smith’s Textbook of Endourology. Hoboken, NJ: Wiley-Blackwell. 2012;527–58.
15. Ali ZA, Mc Entegart M, Hill JM, Spratt JC. Intravascular lithotripsy for treatment of stent underexpansion secondary to severe coronary calcification. Eur Heart J. 2020;41(3):485-6. PubMed | CrossRef
16. Ali ZA, Nef H, Escaned J, Werner N, Banning AP, Hill JM, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the disrupt CAD II study. Circ Cardiovasc Interv. 2019;12(10):008434. PubMed
17. Li D, Pellegrino A, Hallack A, Petrinic N, Jérusalem A, Cleveland RO. Response of single cells to shock waves and numerically optimized waveforms for cancer therapy. Biophys J. 2018;114(6):1433-9. PubMed | CrossRef
18. Brodmann M, Werner M, Holden A, Tepe G, Scheinert D, Schwindt A, et al. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: results of Disrupt PAD II. Catheter Cardiovasc Interv. 2019;93(2):335-42. PubMed | CrossRef
19. Kereiakes DJ, Virmani R, Hokama JY, Illindala U, Mena-Hurtado C, Holden A, et al. Principles of intravascular lithotripsy for calcific plaque modification. J Am Coll Cardiol. 2021;14(12):1275-92. PubMed | CrossRef
20. Ali ZA, Brinton TJ, Hill JM, Maehara A, Matsumura M, Karimi Galougahi K, et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovasc Imaging. 2017;10(8):897-906. PubMed | CrossRef
21. Holden A. Safety and performance of the Shockwave Lithoplasty System in treating calcified peripheral vascular lesions: intravascular OCT analysis. Leipzig Germany. 2018. PubMed | CrossRef
22. Isner JM, Donaldson RF, Fortin AH, Tischler A, Clarke RH. Attenuation of the media of coronary arteries in advanced atherosclerosis. Am J Cardiol. 1986;58(10):937-9.9. PubMed | CrossRef
23. Madhavan MV, Shahim B, Mena‐Hurtado C, Garcia L, Crowley A, Parikh SA. Efficacy and safety of intravascular lithotripsy for the treatment of peripheral arterial disease: An individual patient‐level pooled data analysis.mCatheter Cardiovasc Interv. 2020;95(5):959-68. PubMed
24. Brinton TJ, Ali ZA, Hill JM, Meredith IT, Maehara A, Illindala U, et al. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses: first description. Circulation. 2019;139(6):834-6. PubMed | CrossRef
25. Blachutzik F, Honton B, Escaned J, Hill JM, Werner N, Banning AP, et al. Safety and effectiveness of coronary intravascular lithotripsy in eccentric calcified coronary lesions: a patient-level pooled analysis from the Disrupt CAD I and CAD II Studies. Clin Res Cardiol. 2021;110(2):228-36. PubMed | CrossRef
26. Tepe G, Brodmann M, Werner M, Bachinsky W, Holden A, Zeller T, et al. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized Disrupt PAD III trial.
Circ CardiovascInterv. 2021;14(12):1352-61. CrossRef
27. Brodmann M, Werner M, Brinton TJ, Illindala U, Lansky A, Jaff MR, et al. Safety and performance of lithoplasty for treatment of calcified peripheral artery lesions. J Am Coll Cardiol. 2017;70(7):908-10. PubMed | CrossRef
28. Cai WJ, Koltai S, Kocsis E, Scholz D, Kostin S, Luo X, et al. Remodeling of the adventitia during coronary arteriogenesis. Am J Physiol Heart Circ Physiol. 2003;284(1):31-40. PubMed | CrossRef
29. Pasterkamp G, Galis ZS, De Kleijn DP. Expansive arterial remodeling: location, location, location. ArteriosclerThrombVasc Bio. 2004;24(4):650-7. PubMed | CrossRef
30. Michel JB, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A. Topological determinants and consequences of adventitial responses to arterial wall injury. ArteriosclerThrombVasc Biol. 2007;27(6):1259-68. PubMed | CrossRef
31. Xu F, Ji J, Li L, Chen R, Hu W. Activation of adventitial fibroblasts contributes to the early development of atherosclerosis: a novel hypothesis that complements the “Response-to-Injury Hypothesis” and the “Inflammation Hypothesis”. Med Hypotheses. 2007;69(4):908-12. PubMed | CrossRef
32. Wang X, Matsumura M, Mintz GS, Lee T, Zhang W, Cao Y, et al. In vivo calcium detection by comparing optical coherence tomography, intravascular ultrasound, and angiography. JACC Cardiovasc Imaging. 2017;10(8):869-79. PubMed | CrossRef
33. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-42. PubMed | CrossRef
34. Di Mario C, Goodwin M, Ristalli F, Ravani M, Meucci F, Stolcova M, et al. A prospective registry of intravascular lithotripsy-enabled vascular access for transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12(5):502-4. PubMed | CrossRef
35. Rosseel L, De Backer O, Søndergaard L, Bieliauskas G. Intravascular iliac artery lithotripsy to enable transfemoral thoracic endovascular aortic repair. Catheter Cardiovasc Interv. 2020;95(3):96-9. PubMed | CrossRef
36. Riley RF, Corl JD, Kereiakes DJ. Intravascular lithotripsy‐assistedImpella insertion: A case report. Catheter Cardiovasc Interv. 2019;93(7):1317-9. PubMed | CrossRef
37. Saito S, Yamazaki S, Takahashi A, Namiki A, Kawasaki T, Otsuji S, et al. Intravascular Lithotripsy for Vessel Preparation in Severely Calcified Coronary Arteries Prior to Stent Placement-Primary Outcomes From the Japanese Disrupt CAD IV Study. Circ J. 2021;85(6):826-833. PubMed | CrossRef
38. Riley RF, Henry TD, Mahmud E, Kirtane AJ, Brilakis ES, Goyal A, et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter Cardiovasc Interv. 2020;96(2):346-62. PubMed | CrossRef
39. Abdel Wahab M, Toelg R, Byrne RA, Geist V, Robinson DR, Abdelghani M, et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions: the randomized prepare-CALC trial. Circ Cardiovasc Interv. 2018;11(10)007415. PubMed | CrossRef
40. Chambers JW, Feldman RL, Himmelstein SI, Bhatheja R, Villa AE, Strickman NE, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7(5):510-8. PubMed | CrossRef
41. Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high‐energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62(2):155-61. PubMed | CrossRef
Rohit Mody1*, Debabrata Dash2, Bhavya Mody3, Anand Reddy Maligireddy4, Ankit Agarwal5, and Lakshay Rastogi6
1Department of Cardiology, MD, DM, MAX Super specialty hospital, Bathinda, Punjab, India
2Department of Cardiology, MD, DM, FICC, FAPSC, FCCP, FSCAI, Aster Hospital, Dubai, Al Quasis, UAE
3Department of Medicine, MBBS, Kasturba Medical College, Manipal, Karnataka, India
4Department of Cardiology, Mayo Clinic, 13400 E Shea Blvd, Scottsdale, AZ 85259, USA
5Department of Cardiology, Cleveland Clinic, 9500 Euclid Avenue Cleveland, Ohio 44195, USA
6Department of Cardiology, Kasturba Medical College, Manipal, India
*Corresponding Author: Rohit Mody, Department of Cardiology, MD, DM, MAX Super specialty hospital, Bathinda, Punjab, India.
Copyright© 2022 by Mody R, et al. All rights reserved. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mody R, Dash D, Mody B, Maligireddy AR, Agarwal A, Rastogi L. Re-analysis of Coronary Intravascular Lithotripsy - Can It Be Used in All Comers? J Cardiol Cardiovasc Res. 2022;3(1):1-12. DOI: https://doi.org/10.37191/Mapsci-JCCR-3(1)-053
