Abstract
We fabricated (Ph4P)2ZnCl4 and (Ph4P)2ZnBr4 crystals (Ph4P: tetraphenylphosphonium) with zero-dimensional structures via solvent evaporation and evaluated their optical and scintillation properties. Fluorescence and phosphorescence peaks derived from Ph4P+ cations appeared at 345 and 510 nm, respectively, for both crystals, while an emission originating from self-trapped excitons was also detected for (Ph4P)2ZnBr4. In the scintillation spectra, both samples exhibited a phosphorescence peak, while a weak fluorescence peak was also observed for (Ph4P)2ZnCl4. In addition, the scintillation light yield (LY) was determined by pulse-height spectra with 1 μs shaping time. A pulse-height peak was observed from (Ph4P)2ZnCl4, and the scintillation LY was about 670 photons/5.5 MeV-α, which was higher than that of a ZnO single crystal.
Export citation and abstract BibTeX RIS
1. Introduction
Scintillators are a type of phosphor that absorb energy from ionizing radiation and rapidly emit multiple low-energy photons. 1) They have been applied in fields as varied as environmental monitoring, health physics, astrophysics, security, resource exploration, and medical imaging. 2–6) The performance requirements of scintillators vary from application to application, but typically include high light yield (LY), fast decay time, chemical stability, and high density. 7,8) Currently, scintillators in the form of crystals, plastics, and liquids have been developed; 9–19) however, because no single scintillator can meet the requirements of all applications, a suitable one will need to be selected. Till date, many inorganic single crystals (e.g. Bi4Ge3O12, PbWO4, and SrI2: Eu2+) have been used in practice. 20–22)
In recent years, organic–inorganic hybrid compounds (OIHCs) have attracted extensive interest because of their unique emission characteristics. OIHCs consist of organic cations and inorganic halide anions. OIHCs can form zero, one, two, and three-dimensional structures at the nanoscale level (0D, 1D, 2D, and 3D, respectively), depending on the precursors used in the formulation. 23–25) To date, OIHCs with a 2D quantum nanostructure (RNH3)2PbX4 (R: hydrocarbon, X: halide) have been studied as scintillators because they show efficient exciton emissions from the inorganic layer as a result of quantum confinement. 26,27) In particular, the (C6H5C2H4NH3)2PbBr4 crystal exhibits free exciton emissions with a scintillation LY of 14 000 photons MeV−1 and a fast decay time of 11 ns. 28)
In addition, X-ray response properties of a few OIHCs with 0D or 1D structures have been reported. 29,30) For instance, a TEA2Cu2Br4 (TEA: tetraethylammonium) crystal with 0D structure showed a broadband emission that was attributable to self-trapped excitons (STEs), and the scintillation LY was approximately 7600 photons MeV−1. 31) In many cases, an STE emission can be observed from the inorganic part of an OIHC that has 0D or 1D quantum structure, and self-absorption may be suppressed if the Stokes shift is large in comparison to the free exciton emissions. 31–33) However, as scintillators, the properties of OIHCs having 0D or 1D quantum structures remain to be evaluated.
In this study, we focused on (Ph4P)2ZnX4 (Ph4P = tetraphenylphosphonium; X = Cl or Br) with a 0D structure. In this compound, tetrahedral [ZnX4]2− anions form a 0D structure, with the anions separated by Ph4P+ cation spacers. 34) In previous studies, STE emissions were reported from the inorganic [ZnX4]2− ions under UV light. For example, (TMPDA)ZnBr4 (TMPDA: N,N,2,2-tetramethyl-1,3-propanediamine) with a 0D structure shows STE emission from its [ZnBr4]2− component at 452 nm with a decay time of 0.15 μs. 35) It has also been reported that luminescence can be observed from Ph4P+ cations in the (Ph4P)2ZnX4, although their primary role is as spacers: in a previous study, a fluorescence peak at approximately 350 nm and a phosphorescence peak at approximately 500 nm from Ph4PX were observed for (Ph4P)2ZnX4 under 320 nm light. 34) As mentioned above, the photoluminescence (PL) characteristics of (Ph4P)2ZnX4 have been evaluated, but their scintillation properties have not been reported. In this study, (Ph4P)2ZnX4 crystals were prepared by solvent diffusion and their optical and scintillation properties were evaluated.
2. Experimental methods
Tetraphenylphosphonium chloride (Ph4PCl) (Tokyo Chemical Industry Co.), tetraphenylphosphonium bromide (Ph4PBr) (Tokyo Chemical Industry Co.), zinc chloride (99.9%, High Purity Chemicals) and zinc bromide (99.99%, High Purity Chemicals) were used as the precursors. To prepare each sample (X = Cl or Br), stoichiometric amounts of tetraphenylphosphonium halide and the corresponding zinc halide were mixed in ethanol (EtOH) at 70 °C in a tube bottle for 2 h. The tube bottles were stored at 30 °C in an incubator. After one month, (Ph4P)2ZnX4 crystals were found at the bottom of the bottles following gradual evaporation of the EtOH.
X-ray diffraction (XRD) patterns of (Ph4P)2ZnX4 crystals were observed using a diffractometer (RINT–2200 V, Rigaku) in the 2-theta range from 5° to 60°. The XRD patterns of (Ph4P)2ZnX4 were simulated using VESTA software. 36) Emission maps and PL quantum yields (QYs) were measured using a Quantaurus-QY absolute quantum yield spectrometer (Hamamatsu Photonics), and PL decay curves were obtained using a Quantaurus-Tau fluorescence lifetime spectrometer (Hamamatsu Photonics). The scintillation spectra of (Ph4P)2ZnX4 crystals were recorded at RT under X-rays with a CCD spectrometer (DU-420-BU2 spectrometer, Andor) and an optical fiber. 37) The scintillation decay time profiles were recorded using our original evaluation system, previously described. 38) The pulse-height spectra under 5.5 MeV α-radiation from 241Am were recorded using our original system with a photomultiplier tube (PMT) (R7600-U200 Hamamatsu Photonics). 37)
3. Results and discussion
The appearance of (Ph4P)2ZnX4 crystals (X = Cl or Br) is shown in Fig. 1; thicknesses of 2 mm were obtained. Both crystals appeared translucent.
Fig. 1. Appearance of (Ph4P)2ZnX4 crystals (X = Cl, Br).
Download figure:
Standard image High-resolution imageFigure 2 shows the XRD patterns of the (Ph4P)2ZnX4 powders. The diffraction peaks were observed from their compounds in the 2-theta range of 5°–60°. These patterns are in agreement with expected patterns for (Ph4P)2ZnX4, based on simulations from X-ray crystallographic data, 34,36) demonstrating that (Ph4P)2ZnX4 compounds were obtainable by the solvent diffusion process used.
Fig. 2. XRD patterns of (Ph4P)2ZnX4 (X = Cl, Br) and simulated patterns based on Ref. 34.
Download figure:
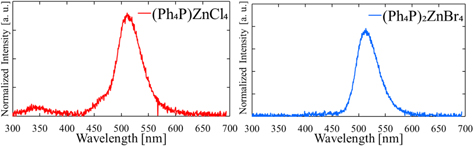
Standard image High-resolution imageFigure 3 shows emission maps of (Ph4P)2ZnX4. Peaks at 345 and 510 nm are observed for both crystals (X = Cl and Br) under 320 nm light. In a previous study, the peaks at 345 and 510 nm were attributed to fluorescence and phosphorescence from Ph4P+ cations, respectively. 34,39,40) In the case of (Ph4P)2ZnBr4, further PL peaks at 430 and 570 nm are observed; the peak at 430 nm was attributed to STE emissions from [ZnBr4]2−. 35,41,42) The origin of the peak at 570 nm is unclear and might be associated with unknown defects or STE. Furthermore, QYs of (Ph4P)2ZnX4 have been reported for 320 and 365 nm light; for 320 nm, the QYs were 6.7% for (Ph4P)2ZnCl4 and 5.9% for (Ph4P)2ZnBr4, while for 365 nm, the QY of (Ph4P)2ZnBr4 was 10.0%.
Fig. 3. PL excitation and emission maps of (Ph4P)2ZnX4 (X = Cl, Br).
Download figure:
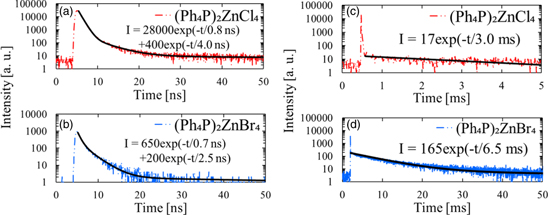
Standard image High-resolution imageThe PL decay time profiles of (Ph4P)2ZnX4 in the nanosecond range under 280 nm light excitation are shown in Figs. 4(a) and 4(b); the decay curves were obtained by monitoring the emission at 315 nm. Two exponential decay components are observed, and the decay times are 0.8 and 4.0 ns for (Ph4P)2ZnCl4 and 0.7 and 2.5 ns for (Ph4P)2ZnBr4. The faster component was likely shorter but limited by the instrumental response, 43) while the slower component was attributed to fluorescence from the Ph4P+ cation. 34,39,40) In addition, the PL decay time profiles of (Ph4P)2ZnX4 in the millisecond range under 315 nm light are shown in Figs. 4(c) and 4(d); the monitoring wavelength was 500 nm. The decay times are 3.0 ms for (Ph4P)2ZnCl4 and 6.5 ms for (Ph4P)2ZnBr4. These were ascribed to phosphorescence from the Ph4P+ cation. 34,39,44) The decay times under 365 nm excitation were also investigated; the decay curves for (Ph4P)2ZnBr4 are shown in Figs. 5(a) and 5(b). The decay time when monitored at 430 nm is 64 ns. The decay times have been attributed to STEs from the inorganic [ZnBr4]2− ions. 35,45,46) Furthermore, the decay times of the first and second components are 90 and 220 ns when monitored at 565 nm.
Fig. 4. PL decay time profiles under 280 nm excitation for (a) (Ph4P)2ZnCl4 and (b) (Ph4P)2ZnBr4. (Note the nanosecond time range) PL decay times under 315 nm excitation in the millisecond range for (c) (Ph4P)2ZnCl4 and (d) (Ph4P)2ZnBr4.
Download figure:
Standard image High-resolution imageFig. 5. PL decay curves of (Ph4P)2ZnBr4 under 365 nm light, monitored at (a) 430 nm, and at (b) 565 nm.
Download figure:
Standard image High-resolution imageFigure 6 shows the X-ray-induced scintillation spectrum of (Ph4P)2ZnX4. Two scintillation bands are observed at 345 and 512 nm for (Ph4P)2ZnCl4 and a single band is observed at 515 nm for (Ph4P)2ZnBr4. By comparison to the PL emission spectra, the broad peak at 345 nm is attributed to fluorescence from Ph4P+ cations, and the broad peaks at 512 and 515 nm are attributed to phosphorescence. 34,40) No STE emission peak of [ZnBr4]2− is observed for (Ph4P)2ZnBr4.
Fig. 6. X-ray induced scintillation spectra of (Ph4P)2ZnX4 (X = Cl, Br).
Download figure:
Standard image High-resolution imageThe scintillation decay curves in the nanosecond range of (Ph4P)2ZnX4 under pulsed X-rays are shown in Figs. 7(a) and 7(b). The decay times of (Ph4P)2ZnCl4 and (Ph4P)2ZnBr4 are 15 ns and 13 ns, respectively; these fast decay components are the result of fluorescence from Ph4P+ cations. 34,39,40) In the scintillation spectra in Fig. 6, no fluorescence peak is detected for (Ph4P)2ZnBr4, but the scintillation decay component of the fluorescence is observed in Fig. 7(b). This inconsistency may result from the scintillation decay curves being recorded by counting individual photons using a photomultiplier tube, a more sensitive technique than using a CCD detector with the scintillation spectrometer. In the millisecond range, the scintillation decay curves of (Ph4P)2ZnX4 are shown in Figs. 7(c) and 7(d); the decay curves are exponential with decay times of 0.10 ms for (Ph4P)2ZnCl4 and 0.65 ms for (Ph4P)2ZnBr4. This decay component originates in the phosphorescence of the Ph4P+ cations. 34,40,44) In comparison to Figs. 4(c) and 4(d), the scintillation decay times for phosphorescence are shorter than those of PL. Generally, scintillation decay times are longer than PL times because of additional excitation processes during scintillation. One possible mechanism might be a high excitation density in scintillation, leading to additional quenching processes such as triplet–triplet annihilation and shorter decay times.
Fig. 7. Pulsed-X-ray-induced scintillation decay time profiles in the nanosecond range for (a) (Ph4P)2ZnCl4 and (b) (Ph4P)2ZnBr4. Pulsed-X-ray-induced scintillation decay time profiles in the millisecond range for (c) (Ph4P)2ZnCl4 and (d) (Ph4P)2ZnBr4.
Download figure:
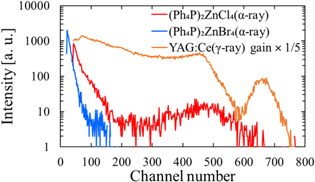
Standard image High-resolution imageFigure 8 shows the pulse-height spectra under 241Am α-particle excitation with 1 μs shaping time. A cerium-activated Y3Al5O12 (YAG:Ce3+) scintillator crystal was employed for reference to calculate a scintillation LY; the LY of YAG:Ce3+ was 20 000 photons MeV−1. 47) It should be noted that the shaping time used was 1 μs; thus, the fluorescence from Ph4P+ cations is the main contributor to the LY. In the pulse-height spectra, peaks were observed at channels 492 for (Ph4P)2ZnCl4 and 660 for YAG:Ce3+, whereas no peak was detected for (Ph4P)2ZnBr4. Quantum efficiencies of the PMT in this system were 14% at 520 nm (YAG:Ce) and 41% at 345 nm ((Ph4P)2ZnCl4). The LY of (Ph4P)2ZnCl4 was calculated to be 670 photons/5.5 MeV-alpha by considering the quantum efficiency and pulse-height peak. This LY is higher than that of an undoped ZnO crystal. 48,49) As shown in Fig. 8, the scintillation LY of (Ph4P)2ZnBr4 was lower than that of (Ph4P)2ZnCl4, possibly because of the heavy atom effect. 34,50,51) Replacing Cl with Br increases the spin–orbit coupling, consequently increasing the phosphorescence component (Fig. 6). This could explain the lack of an observed pulse-height peak for (Ph4P)2ZnBr4.
Fig. 8. Pulse-height spectra of (Ph4P)2ZnX4 (X = Cl, Br) under α-particle radiation from 241Am (5.5 MeV). A YAG:Ce3+ scintillator is used for reference (shown with expanded vertical scale).
Download figure:
Standard image High-resolution image4. Conclusion
We present synthesis of OIHCs with zero-dimensional structure of (Ph4P)2ZnCl4 and (Ph4P)2ZnBr4 crystals and evaluate their scintillation characteristics. Luminescent peaks attributed to the Ph4P+ cations were observed for both crystals. Moreover, the scintillation decay components of the fluorescence and phosphorescence from the Ph4P+ cations were detected. Further, the scintillation LY of (Ph4P)2ZnCl4 under alpha-particle bombardment was estimated to be 670 photons/5.5 MeV-alpha, whereas the comparable signal from (Ph4P)2ZnBr4 was not observable. The higher scintillation LY of (Ph4P)2ZnCl4 compared to that of (Ph4P)2ZnBr4 may be associated with a heavy-atom effect.
Acknowledgments
This work was supported by Grants-in-Aid (19K20596 and 21H03733) from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government (MEXT).