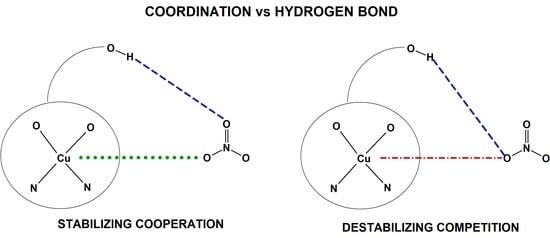
Symmetry in Recognition of Supramolecular Synthons–Competition between Hydrogen Bonding and Coordination Bond in Multinuclear CuII–4f Complexes with Bicompartmental Schiff Base Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Complexes
2.3. Methods
3. Results and Discussion
3.1. Molecular and Crystal Structure of Complexes 1–3
3.2. Hexanuclear Complex of 1
3.3. Dimer of Trinuclear Cores Linked by a Semi-Coordination Bond in 2
3.4. Supramolecular Polymer Built of Hexanuclear Monomers in 3
3.5. The Geometric Criteria for a Semi-Coordination Bond on the Base of CSD Search
3.6. Infrared Spectra
3.7. Thermal Properties
3.8. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sundberg, M.R. Effect of hydrogen bonding on coordinate bond. Semi-coordination in Werner-type copper(II) complexes revisited. Rev. Inorg. Chem. 2000, 20, 195–218. [Google Scholar] [CrossRef]
- Grabowski, S.; Grabowski, J.S. Analysis of Hydrogen Bonds in Crystals. Crystals 2016, 6, 59. [Google Scholar] [CrossRef]
- Videnova-Adrabińska, V. The hydrogen bond as a design element in crystal engineering. Two- and three-dimensional building blocks of crystal architecture. J. Mol. Struct. 1996, 374, 199–222. [Google Scholar]
- Aakeröy, C.B.; Seddon, K.R. The hydrogen bond and crystal engineering. Chem. Soc. Rev. 1993, 22, 397–407. [Google Scholar] [CrossRef]
- Desiraju, G.R. Reflections on the Hydrogen Bond in Crystal Engineering. Cryst. Growth Des. 2011, 11, 896–898. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Miroslaw, B.; Koziol, A.E.; Bielenica, A.; Dziuba, K.; Struga, M. Substituent effect on supramolecular motifs in series of succinimide polycyclic keto derivatives—Spectroscopic, theoretical and crystallographic studies. J. Mol. Struct. 2014, 1074, 695–702. [Google Scholar] [CrossRef]
- Ding, X.; Tuikka, M.; Haukk, M. Halogen Bonding in Crystal Engineering. In Recent Advances in Crystallography; Benedict, J.B., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [Green Version]
- Miroslaw, B.; Plech, T.; Wujec, M. Halogen bonding in the antibacterial 1,2,4-triazole-3-thione derivative - Spectroscopic properties, crystal structure and conformational analysis. J. Mol. Struct. 2015, 1083, 187–193. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Struga, M.; Miroslaw, B.; Pakosinska-Parys, M.; Drzewiecka, A.; Borowski, P.; Kossakowski, J.; Koziol, A.E. Synthesis, characterization and supramolecular synthons in crystals of new derivatives of 10-oxa-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. J. Mol. Struct. 2010, 965. [Google Scholar] [CrossRef]
- Madura, I.D.; Czerwińska, K.; Jakubczyk, M.; Pawełko, A.; Adamczyk-Woźniak, A.; Sporzyński, A. Weak C–H⋯O and Dipole–Dipole Interactions as Driving Forces in Crystals of Fluorosubstituted Phenylboronic Catechol Esters. Cryst. Growth Des. 2013, 13, 5344–5352. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Sarma, J.A.R.P.; Krishna, T.S.R. Dipole-dipole interactions and the inversion motif in the crystal structures of planar chloro aromatics: The unusual packings of 1,2,3-trichlorobenzene and 1,2,3,7,8,9-hexachlorodibenzo-p-dioxin. Chem. Phys. Lett. 1986, 131, 124–128. [Google Scholar] [CrossRef]
- Newman, W.D.; Cortes, C.L.; Afshar, A.; Cadien, K.; Meldrum, A.; Fedosejevs, R.; Jacob, Z. Observation of long-range dipole-dipole interactions in hyperbolic metamaterials. Sci. Adv. 2018, 4, eaar5278. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, G.; Bauzá, A.; Gurbanov, A.V.; Zubkov, F.I.; Maniukiewicz, W.; Rodríguez-Diéguez, A.; López-Torres, E.; Frontera, A. The role of unconventional stacking interactions in the supramolecular assemblies of Hg(ii) coordination compounds. CrystEngComm 2016, 18, 9056–9066. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Zaręba, J.K.; Bauzá, A.; Kubicki, M.; Bartyzel, A.; Keramidas, A.D.; Butusov, L.; Mirosław, B.; Frontera, A. Recurrent supramolecular motifs in discrete complexes and coordination polymers based on mercury halides: prevalence of chelate ring stacking and substituent effects. CrystEngComm 2018, 20, 1065–1076. [Google Scholar] [CrossRef]
- James, S. Pi-Pi-Stacking as a Crystal Engineering Tool. In Encyclopedia of Supramolecular Chemistry; Atwood, J.L., Steed, J.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 1093–1099. [Google Scholar]
- Yao, Z.-F.; Wang, J.-Y.; Pei, J. Control of π–π Stacking via Crystal Engineering in Organic Conjugated Small Molecule Crystals. Cryst. Growth Des. 2018, 18, 7–15. [Google Scholar] [CrossRef]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Blagojević Filipović, J.P.; Hall, M.B.; Zarić, S.D. Stacking interaction potential energy surfaces of square-planar metal complexes containing chelate rings. Adv. Inorg. Chem. 2019, 73, 159–186. [Google Scholar]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Doyle, R.P.; Julve, M.; Lloret, F.; Nieuwenhuyzen, M.; Kruger, P.E. Hydrogen-bond tuning of ferromagnetic interactions: synthesis, structure and magnetic properties of polynuclear copper(ii) complexes incorporating p-block oxo-anions. Dalt. Trans. 2006, 0, 2081–2088. [Google Scholar] [CrossRef]
- Tang, J.; Costa, J.S.; Golobic, A.; Kozlevcar, B.; Robertazzi, A.; Vargiu, A.V.; Gamez, P.; Reedijk, J. Magnetic coupling between copper(II) ions mediated by hydrogen-bonded (neutral) water molecules. Inorg. Chem. 2009, 48, 5473–5479. [Google Scholar] [CrossRef] [PubMed]
- Mobin, S.M.; Srivastava, A.K.; Mathur, P.; Lahiri, G.K. Varying structural motifs in oxyanions (NO3−, CO32−) and phenoxyacetate (PhOAc−) bridged coordination polymers derived from alkoxo-bridged dicopper building blocks with {Cu2O2} core. RSC Adv. 2011, 1, 893–902. [Google Scholar] [CrossRef]
- Haldar, S.; Vijaykumar, G.; Carrella, L.; Musie, G.T.; Bera, M. Structure and properties of a novel staircase-like decanuclear [CuII10] cluster supported by carbonate and carboxylate bridges. New J. Chem. 2018, 42, 1276–1283. [Google Scholar] [CrossRef]
- Takeda, S.; Watanabe, A.; Maruta, G.; Matsuo, T. Magnetic Interactions through Short Hydrogen Bond in Hydrogen-Bonded Basic Copper (II) Salt; Cu 2 Na(D 3 O 2 )(SO 4 ) 2. Mol. Cryst. Liq. Cryst. 2002, 376, 443–448. [Google Scholar] [CrossRef]
- Brown, D.S.; Lee, J.D.; Melsom, B.G.A.; Hathaway, B.J.; Procter, I.M.; Tomlinson, A.A.G. The structure of Cu(en)2(BF4)2 and an infrared spectral criterion for “semi-co-ordinated” polyanions. Chem. Commun. (London) 1967, 0, 369–371. [Google Scholar] [CrossRef]
- Valach, F. A bond-valence approach to the semicoordination of copper-oxygen and copper–nitrogen complexes. Polyhedron 1999, 18, 699–706. [Google Scholar] [CrossRef]
- Valach, F.; Rohlíček, J.; Lukeš, V.; Kožíšek, J.; Jorík, V. Manifestation of copper coordination sphere plasticity in [Cu2(2-bromopropionato)4]n and [Cu2(3-bromopropionato)4(H2O)2]. Inorganica Chim. Acta 2018, 479, 106–112. [Google Scholar] [CrossRef]
- Choi, J.-H.; Joshi, T.; Spiccia, L. Syntheses, Structural, and Spectroscopic Properties of Copper(II) Complexes of Constrained Macrocyclic Ligands. Zeitschrift für Anorg. und Allg. Chemie 2012, 638, 146–151. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Electrophilic–Nucleophilic Dualism of Nickel(II) toward Ni⋯I Noncovalent Interactions: Semicoordination of Iodine Centers via Electron Belt and Halogen Bonding via σ-Hole. Inorg. Chem. 2017, 56, 13562–13578. [Google Scholar] [CrossRef]
- Valach, F.; Grobelny, R.; Glowiak, T.; Mrozinski, J.; Lukeš, V.; Blahová, Z. Structural study of semi-coordination in a seven-coordinate copper(II) complex: distortion isomerism of [Cu(CH3COO)2 (4-aminopyridine)2 (H2O)]. J. Coord. Chem. 2010, 63, 1645–1651. [Google Scholar] [CrossRef]
- Nimmermark, A.; Öhrström, L.; Reedijk, J. Metal-ligand bond lengths and strengths: are they correlated? A detailed CSD analysis. Zeitschrift für Krist. - Cryst. Mater. 2013, 228, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Nelyubina, Y.V.; Korlyukov, A.A.; Fedyanin, I.V.; Lyssenko, K.A. Extremely long Cu⋯O contact as a possible pathway for magnetic interactions in Na2Cu(CO3)2. Inorg. Chem. 2013, 52, 14355–14363. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular Polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Hager, M.D.; Schubert, U.S. Supramolecular Polymers. Polym. Sci. A Compr. Ref. 2012, 269–310. [Google Scholar]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef] [PubMed]
- Krieg, E.; Bastings, M.M.C.; Besenius, P.; Rybtchinski, B. Supramolecular Polymers in Aqueous Media. Chem. Rev. 2016, 116, 2414–2477. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, B.; Miroslaw, B.; Bartyzel, A. Hexanuclear [Cu4IILn2III] compounds incorporating N,O-donor ligands—Synthesis, crystal structures and physicochemical properties. Inorganica Chim. Acta 2017, 466, 160–165. [Google Scholar] [CrossRef]
- Cristóvão, B.; Osypiuk, D.; Miroslaw, B.; Bartyzel, A. Syntheses, crystal structures, thermal and magnetic properties of new heterotrinuclear CuII–LnIII–CuII complexes incorporating N2O4-donor Schiff base ligands. Polyhedron 2018, 144, 225–233. [Google Scholar] [CrossRef]
- Bermejo, M.R.; Fernández, M.I.; Gómez-Fórneas, E.; González-Noya, A.; Maneiro, M.; Pedrido, R.; Rodríguez, M.J. Self-Assembly of Dimeric MnIII–Schiff-Base Complexes Tuned by Perchlorate Anions. Eur. J. Inorg. Chem. 2007, 2007, 3789–3797. [Google Scholar] [CrossRef]
- Aguiari, A.; Bullita, E.; Casellato, U.; Guerriero, P.; Tamburini, S.; Vigato, P.A. Macrocyclic and macroacyclic compartmental Schiff bases: synthesis, characterization, X-ray structure and interaction with metal ions. Inorganica Chim. Acta 1992, 202, 157–171. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Rigaku Oxford Diffraction Ltd. Crysalis-Pro Software System; ver. 1.171.38.46; Rigaku Corporation Ltd.: Oxford, UK, 2016. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. IUCr New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. Diamond; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Li, G.-B.; Hong, X.-J.; Gu, Z.-G.; Zheng, Z.-P.; Wu, Y.-Y.; Jia, H.-Y.; Liu, J.; Cai, Y.-P. Efficient synthesis and characterization of the low dimensional heteronuclear complexes with a N2O2-donor Schiff base ligand. Inorganica Chim. Acta 2012, 392, 177–183. [Google Scholar] [CrossRef]
- Alaghaz, A.-N.M.A.; El-Sayed, B.A.; El-Henawy, A.A.; Ammar, R.A.A. Synthesis, spectroscopic characterization, potentiometric studies, cytotoxic studies and molecular docking studies of DNA binding of transition metal complexes with 1,1-diaminopropane–Schiff base. J. Mol. Struct. 2013, 1035, 83–93. [Google Scholar] [CrossRef]
- Dubey, R.K.; Baranwal, P.; Jha, A.K. Zinc(II) and mercury(II) complexes with Schiff bases: syntheses, spectral, and structural characterization. J. Coord. Chem. 2012, 65, 2645–2656. [Google Scholar] [CrossRef]
- Bartyzel, A. Synthesis, crystal structure and characterization of manganese(III) complex containing a tetradentate Schiff base. J. Coord. Chem. 2013, 66, 4292–4303. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Perpiñán, M.F.; Sánchez, A.E.; Torralba, M.C.; González, V. Water inclusion mediated structural diversity and the role of H-bonds in molecular assemblies of manganese(III) bicompartmental Schiff-base complexes. Inorganica Chim. Acta 2016, 453, 169–178. [Google Scholar] [CrossRef]













| Identification Code | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C76H82N14O35Cu4Tm2 | C76H88N14O38Cu4Ho2 | C156H184N28O76Cu8Er4 |
| Formula weight | 2343.57 | 2389.62 | 4844.66 |
| Temperature/K | 294.1(3) | 120.0(0) | 120.0(1) |
| Crystal system | monoclinic | monoclinic | orthorhombic |
| Space group | C2/c | P21/c | Pnc2 |
| a/Å | 26.043(1) | 26.134(1) | 16.8980(4) |
| b/Å | 15.0653(4) | 15.5412(6) | 33.1478(7) |
| c/Å | 22.7584(9) | 21.515(1) | 15.5493(3) |
| α/° | 90 | 90 | 90 |
| β/° | 101.659(4) | 94.386(5) | 90 |
| γ/° | 90 | 90 | 90 |
| Volume/Å3 | 8744.9(5) | 8712.5(7) | 8709.6(3) |
| Z | 4 | 4 | 2 |
| ρcalc g/cm3 | 1.780 | 1.822 | 1.847 |
| μ/mm‑1 | 5.508 | 5.149 | 5.342 |
| F(000) | 4680.0 | 4784.0 | 4856.0 |
| Crystal size/mm3 | 0.22 × 0.2 × 0.15 | 0.2 × 0.1 × 0.05 | 0.25 × 0.08 × 0.02 |
| Radiation | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) |
| 2Θ range for data collection/° | 6.814 to 135.366 | 6.622 to 135.364 | 7.472 to 153.146 |
| Index ranges | −31 ≤ h ≤ 25, −17 ≤ k ≤ 18, −27 ≤ l ≤ 26 | −31 ≤ h ≤ 31, −18 ≤ k ≤ 14, −25 ≤ l ≤ 25 | −21 ≤ h ≤ 18, −30 ≤ k ≤ 41, −19 ≤ l ≤ 18 |
| Reflections collected | 29701 | 60265 | 65209 |
| Independent reflections | 7897 [Rint = 0.0434, Rsigma = 0.0366] | 15748 [Rint = 0.1026, Rsigma = 0.0951] | 17487 [Rint = 0.0537, Rsigma = 0.0509] |
| Data/restraints/parameters | 7897/40/586 | 15748/36/1218 | 17487/37/1220 |
| Goodness-of-fit on F2 | 1.058 | 0.984 | 1.033 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0596, wR2 = 0.1696 | R1 = 0.0599, wR2 = 0.1498 | R1 = 0.0425, wR2 = 0.1076 |
| Final R indexes [all data] | R1 = 0.0727, wR2 = 0.1922 | R1 = 0.0978, wR2 = 0.1788 | R1 = 0.0529, wR2 = 0.1333 |
| Largest diff. peak/hole/e Å−3 | 1.33/−1.13 | 1.36/−1.33 | 1.12/−1.32 |
| Flack parameter | – | – | 0.001(5) |
| CCDC No. | 1901698 | 1901699 | 1901700 |
| 1 | 2 | 3 | |||
|---|---|---|---|---|---|
| Tm1–Cu1 | 3.463(1) | Ho1–Cu1 | 3.455(1) | Er1–Cu1 | 3.443(2) |
| Tm1–Cu2 | 3.4636(9) | Ho1–Cu2 | 3.454(1) | Er1–Cu2 | 3.473(2) |
| Tm1–O1 | 2.280(5) | Ho1–O1 | 2.288(4) | Er1–O1 | 2.296(7) |
| Tm1–O2 | 2.317(4) | Ho1–O2 | 2.298(4) | Er1–O2 | 2.283(6) |
| Tm1–O3 | 2.289(5) | Ho1–O3 | 2.408(5) | Er1–O3 | 2.377(7) |
| Tm1–O4 | 2.377(5) | Ho1–O4 | 2.355(4) | Er1–O4 | 2.363(7) |
| Tm1–O5 | 2.291(5) | Ho1–O5 | 2.294(5) | Er1–O5 | 2.297(7) |
| Tm1–O6 | 2.306(4) | Ho1–O6 | 2.300(5) | Er1–O6 | 2.324(7) |
| Tm1–O7 | 2.326(6) | Ho1–O7 | 2.379(5) | Er1–O7 | 2.251(7) |
| Tm1–O8 | 2.368(5) | Ho1–O8 | 2.348(5) | Er1–O8 | 2.412(6) |
| Cu1–O1 | 1.934(5) | Ho2–Cu3 | 3.513(1) | Er2–Cu3 | 3.474(2) |
| Cu1–O2 | 1.941(4) | Ho2–Cu4 | 3.491(1) | Er2–Cu4 | 3.449(2) |
| Cu1–N1 | 1.983(5) | Ho2–O9 | 2.354(5) | Er2–O9 | 2.301(7) |
| Cu1–N2 | 1.987(6) | Ho2–O10 | 2.321(5) | Er2–O10 | 2.312(7) |
| Cu1–O17 | 2.57(2) | Ho2–O11 | 2.421(5) | Er2–O11 | 2.355(7) |
| Cu1⋯O16 | 3.57(2) | Ho2–O12 | 2.399(5) | Er2–O12 | 2.388(7) |
| Cu1–O16A | 2.93(2) | Ho2–O13 | 2.359(5) | Er2–O13 | 2.286(7) |
| Cu1–O16B | 2.66(1) | Ho2–O14 | 2.335(5) | Er2–O14 | 2.314(6) |
| Cu1–O17 | 2.57(2) | Ho2–O15 | 2.446(6) | Er2–O15 | 2.238(7) |
| Cu2–O5 | 1.930(5) | Ho2–O16 | 2.481(5) | Er2–O16 | 2.417(7) |
| Cu2–O6 | 1.935(4) | Ho2–O38A | 2.44(1) | Cu1–O1 | 1.959(8) |
| Cu2–N3 | 1.977(5) | Ho2–O38 | 2.372(9) | Cu1–O2 | 1.939(7) |
| Cu2–N4 | 1.975(5) | Cu1–O1 | 1.949(5) | Cu1–N1 | 1.99(1) |
| Cu2–O10 | 2.594(5) | Cu1–O2 | 1.953(5) | Cu1–N2 | 1.99(1) |
| Cu2⋯O11 | 3.28(2) | Cu1–N1 | 1.982(6) | Cu1–O17 | 2.29(1) |
| Cu1–N2 | 1.979(6) | Cu1–O30 | 2.83(1) | ||
| Cu1–O32 | 2.497(6) | Cu2–O5 | 1.90(1) | ||
| Cu1⋯O35 | 3.89(1) | Cu2–O6 | 1.959(7) | ||
| Cu2–O5 | 1.933(5) | Cu2–N3 | 1.995(9) | ||
| Cu2–O6 | 1.939(5) | Cu2–N4 | 1.948(9) | ||
| Cu2–N3 | 1.967(6) | Cu2–O37 | 2.908(9) | ||
| Cu2–N4 | 1.965(6) | Cu2–O18 | 3.19(1)* | ||
| Cu2–O24 | 2.488(5) | Cu3–O9 | 1.922(7) | ||
| Cu2⋯O26 | 3.356(7) | Cu3–O10 | 1.952(7) | ||
| Cu3–O9 | 1.933(5) | Cu3–O21 | 2.44(1) | ||
| Cu3–O10 | 1.960(5) | Cu3–N7 | 1.977(9) | ||
| Cu3–N5 | 1.989(6) | Cu3–N8 | 1.962(9) | ||
| Cu3–N6 | 1.972(6) | Cu3–O21 | 2.44(1) | ||
| Cu3–O25 | 3.031(5) | Cu3⋯O38 | 3.613(8) | ||
| Cu3–O37 | 2.478(8) | Cu4–O13 | 1.915(7) | ||
| Cu4–O13 | 1.953(5) | Cu4–O14 | 1.958(7) | ||
| Cu4–O14 | 1.936(5) | Cu4–N5 | 1.969(9) | ||
| Cu4–N7 | 1.972(6) | Cu4–N6 | 1.965(9) | ||
| Cu4–N8 | 1.972(6) | Cu4–O27 | 2.565(7) | ||
| Cu4–O18 | 2.565(6) | Cu4–O20 | 3.01(1) | ||
| Cu4–O22 | 2.603(5) |
| 1 | 2 | 3 | |||
|---|---|---|---|---|---|
| Cu1–O1–Tm1 | 110.3(2) | Cu1–O1–Ho1 | 109.0(2) | Cu1–O1–Er1 | 107.8(3) |
| Cu1–O2–Tm1 | 108.5(2) | Cu1–O2–Ho1 | 108.5(2) | Cu1–O2–Er1 | 109.0(3) |
| Cu2–O5–Tm1 | 110.0(2) | Cu2–O5–Ho1 | 109.3(2) | Cu2–O5–Er1 | 111.3(3) |
| Cu2–O6–Tm1 | 109.2(2) | Cu2–O6–Ho1 | 108.8(2) | Cu2–O6–Er1 | 108.1(3) |
| Cu1–Tm1–Cu2 | 175.6(1) | Cu3–O9–Ho2 | 109.7(2) | Cu3–O9–Er2 | 109.2(3) |
| Cu3–O10–Ho2 | 110.0(2) | Cu3–O10–Er2 | 107.7(3) | ||
| Cu4–O13–Ho2 | 107.7(2) | Cu4–O13–Er2 | 111.3(3) | ||
| Cu4–O14–Ho2 | 109.3(2) | Cu4–O14–Er2 | 108.6(3) | ||
| Cu1–Ho1–Cu2 | 170.5(1) | Cu1–Er1–Cu2 | 174.3(1) | ||
| Cu3–Ho2–Cu4 | 177.1(1) | Cu3–Er2–Cu4 | 171.3(1) |
| Crystal | D–H⋯A | D–H | H⋯A | D⋯A | ∠D–H⋯A |
|---|---|---|---|---|---|
| 1 | O3–H3⋯O11 | 0.87 | 1.76 | 2.55(2) | 150 |
| O4–H4⋯O9 | 0.87 | 1.88 | 2.614(6) | 143 | |
| O7–H7⋯O17 | 0.87 | 2.26 | 2.87(2) | 128 | |
| O7–H7⋯O19 | 0.87 | 2.26 | 3.00(1) | 144 | |
| O7–H7⋯O20 | 0.87 | 1.80 | 2.64(2) | 165 | |
| O8–H8⋯O14 | 0.87 | 1.61 | 2.54(2) | 161 | |
| O8–H8⋯O14A | 0.87 | 2.02 | 2.89(2) | 150 | |
| O8–H8⋯O16B | 0.87 | 1.67 | 2.59(2) | 157 | |
| 2 | O3–H3⋯O23 | 0.86 | 1.79 | 2.628(7) | 167 |
| O4–H4⋯O26 | 0.86 | 1.69 | 2.535(8) | 167 | |
| O7–H7⋯O32 | 0.86 | 1.75 | 2.545(8) | 154 | |
| O8–H8⋯O35 | 0.86 | 1.74 | 2.544(9) | 158 | |
| O35–H35A⋯O36 i | 0.86 | 2.22 | 2.819(16) | 127 | |
| O11–H11⋯O20 | 0.86 | 1.76 | 2.592(9) | 167 | |
| O12–H12⋯O17 | 0.86 | 1.76 | 2.587(8) | 166 | |
| O15–H15⋯O29 | 0.86 | 1.80 | 2.621(10) | 163 | |
| O16–H16⋯O23 | 0.86 | 1.97 | 2.723(8) | 148 | |
| O38A–H38A⋯O37 ii | 0.89 | 2.23 | 3.04(1) | 152 | |
| O38–H38C⋯O22 ii | 0.89 | 2.64 | 3.14(1) | 118 | |
| O37–H37A⋯O29 | 0.85 | 2.04 | 2.719(15) | 135 | |
| O37–H37B⋯O30 iii | 0.85 | 2.03 | 2.825(15) | 154 | |
| O36–H36B⋯O35 i | 0.89 | 2.49 | 2.819(16) | 103 | |
| O35–H35A⋯O26 iv | 0.85 | 2.018 | 2.83(1) | 159.6 | |
| 3 | O3–H3⋯O19 | 0.86 | 1.78 | 2.61(1) | 162 |
| O4–H4⋯O37 | 0.86 | 1.73 | 2.55(1) | 158 | |
| O7–H7⋯O17 | 0.86 | 1.85 | 2.66(1) | 134 | |
| O8–H8⋯O29 | 0.86 | 1.81 | 2.641(8) | 164 | |
| O11–H11⋯O19 | 0.86 | 2.50 | 3.10(1) | 127 | |
| O11–H11⋯O20 | 0.86 | 1.79 | 2.60(1) | 159 | |
| O12–H12⋯O28 | 0.86 | 1.79 | 2.617(9) | 160 | |
| O15–H15⋯O38 | 0.86 | 1.79 | 2.66(1) | 163 | |
| O16–H16⋯O23 | 0.86 | 1.91 | 2.72(1) | 158 | |
| O17A–H17A⋯O38 | 0.86 | 1.95 | 2.76(1) | 156 | |
| O39–H39A⋯O18 | 0.86 | 2.31 | 2.96(1) | 131 | |
| O37–H37⋯O34 | 0.84 | 1.80 | 2.63(1) | 171 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroslaw, B.; Osypiuk, D.; Cristóvão, B.; Głuchowska, H. Symmetry in Recognition of Supramolecular Synthons–Competition between Hydrogen Bonding and Coordination Bond in Multinuclear CuII–4f Complexes with Bicompartmental Schiff Base Ligand. Symmetry 2019, 11, 460. https://doi.org/10.3390/sym11040460
Miroslaw B, Osypiuk D, Cristóvão B, Głuchowska H. Symmetry in Recognition of Supramolecular Synthons–Competition between Hydrogen Bonding and Coordination Bond in Multinuclear CuII–4f Complexes with Bicompartmental Schiff Base Ligand. Symmetry. 2019; 11(4):460. https://doi.org/10.3390/sym11040460
Chicago/Turabian StyleMiroslaw, Barbara, Dariusz Osypiuk, Beata Cristóvão, and Halina Głuchowska. 2019. "Symmetry in Recognition of Supramolecular Synthons–Competition between Hydrogen Bonding and Coordination Bond in Multinuclear CuII–4f Complexes with Bicompartmental Schiff Base Ligand" Symmetry 11, no. 4: 460. https://doi.org/10.3390/sym11040460