Utilization of Inexpensive Carbon-Based Substrates as Platforms for Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Au Nanostructures via Electrodeposition
2.3. Characterization
3. Results and Discussion
3.1. Characterization of Gold Nanostructures
3.2. Electrodeposited Gold Nanostructures for SERS Application
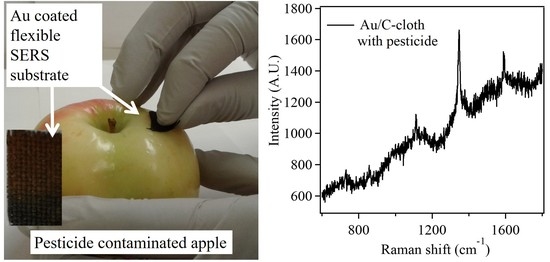
3.3. In-Field Testing Using Gold Nanostructures for Detection of Paraoxon via SERS
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halvorson, R.; Vikesland, P. Surface-Enhanced Raman Spectroscopy (SERS) for Environmental Analyses. Environ. Sci. Technol. 2010, 44, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pino, F.; Merkoci, A. Nanomaterials for Sensing and Destroying Pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, K.; Han, D.; Pang, Q. Surface enhanced Raman scattering (SERS) spectra of trinitrotoluene in silver colloids prepared by microwave heating method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Du, H.; Cheng, F.; Wang, C.; Wang, C.; Fan, M. Fabrication of SERS Swab for Direct Detection of Trace Explosives in Fingerprints. ACS Appl. Mater. Interfaces 2014, 6, 21931–21937. [Google Scholar] [CrossRef] [PubMed]
- Tripp, R.; Dluhy, R.; Zhao, Y. Novel nanostructures for SERS biosensing. Nano Today 2008, 3, 31–37. [Google Scholar] [CrossRef]
- Muehlethaler, C.; Leona, M.; Lombardi, J. Review of Surface Enhanced Raman Scattering Applications in Forensic Science. Anal. Chem. 2016, 88, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Xu, A.; Yeung, E. Determination of NAD(+) and NADH in a Single Cell under Hydrogen Peroxide Stress by Capillary Electrophoresis. Anal. Chem. 2009, 81, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Reisch, A.; Elpeleg, O.; Pon, L.; Schon, E. Biochemical assays for mitochondrial activity: Assays of TCA cycle enzymes and PDHc. Methods Cell Biol. 2007, 80, 199–222. [Google Scholar] [PubMed]
- Patra, D.; Mishra, A. Fluorescence quenching of benzo[k]fluoranthene in poly(vinyl alcohol) film: A possible optical sensor for nitro aromatic compounds. Sens. Actuators B Chem. 2001, 80, 278–282. [Google Scholar] [CrossRef]
- Hill, H.; Simpson, G. Capabilities and limitations of ion mobility spectrometry for field screening applications. Field Anal. Chem. Technol. 1997, 1, 119–134. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.; Mcquillan, A. Raman-spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Lin, X.; Cui, Y.; Xu, Y.; Ren, B.; Tian, Z. Surface-enhanced Raman spectroscopy: Substrate-related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Andrade, G.; Brolo, A. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal. Chim. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jin, R.; Mirkin, C. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Haka, A.; Kneipp, H.; Badizadegan, K.; Yoshizawa, N.; Boone, C.; Shafer-Peltier, K.; Motz, J.; Dasari, R.; Feld, M. Surface-enhanced Raman Spectroscopy in single living cells using gold nanoparticles. Appl. Spectrosc. 2002, 56, 150–154. [Google Scholar] [CrossRef]
- Villa, J.; dos Santos, D.; Poppi, R. Fabrication of gold nanoparticle-coated paper and its use as a sensitive substrate for quantitative SERS analysis. Microchim. Acta 2016, 183, 2745–2752. [Google Scholar] [CrossRef]
- Lee, C.; Hankus, M.; Tian, L.; Pellegrino, P.; Singamaneni, S. Highly Sensitive Surface Enhanced Raman Scattering Substrates Based on Filter Paper Loaded with Plasmonic Nanostructures. Anal. Chem. 2011, 83, 8953–8958. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ren, B.; Li, X.; She, C.; Liu, F.; Cai, X.; Tian, Z. Investigation of surface-enhanced Raman scattering from platinum electrodes using a confocal Raman microscope: Dependence of surface roughening pretreatment. Surf. Sci. 1998, 406, 9–22. [Google Scholar] [CrossRef]
- Brolo, A.; Arctander, E.; Gordon, R.; Leathem, B.; Kavanagh, K. Nanohole-enhanced Raman scattering. Nano Lett. 2004, 4, 2015–2018. [Google Scholar] [CrossRef]
- Min, Q.; Santos, M.; Girotto, E.; Brolo, A.; Gordon, R. Localized Raman enhancement from a double-hole nanostructure in a metal film. J. Phys. Chem. C 2008, 112, 15098–15101. [Google Scholar] [CrossRef]
- Im, H.; Bantz, K.; Lindquist, N.; Haynes, C.; Oh, S. Vertically Oriented Sub-10-nm Plasmonic Nanogap Arrays. Nano Lett. 2010, 10, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Whale, A.; Padalkar, S. Exploring the Efficacy of Platinum and Palladium Nanostructures for Organic Molecule Detection via Raman Spectroscopy. Sensors 2018, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Joshi, P.; Zhou, Y.; Ding, R.; Zhang, P. Quantitative SERS-based DNA detection assisted by magnetic microspheres. Chem. Commun. 2015, 51, 15284–15286. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.; Young, M.; Dieringer, J.; Van Duyne, R. Wavelength-scanned surface-enhanced Raman excitation spectroscopy. J. Phys. Chem. B 2005, 109, 11279–11285. [Google Scholar] [CrossRef] [PubMed]
- Abu Hatab, N.; Oran, J.; Sepaniak, M. Surface-enhanced Raman spectroscopy substrates created via electron beam lithography and nanotransfer printing. ACS Nano 2008, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Chang, S.; Tsukruk, V. Porous Substrates for Label-Free Molecular Level Detection of Nonresonant Organic Molecules. ACS Nano 2009, 3, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tessier, P.; Velev, O.; Kalambur, A.; Lenhoff, A.; Rabolt, J.; Kaler, E. Structured metallic films for optical and spectroscopic applications via colloidal crystal templating. Adv. Mater. 2001, 13, 396–400. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, X.; Yin, D.; Gong, M.; Yang, L.; Zhao, B.; Ruan, W. Rapid monitoring of benzylpenicillin sodium using Raman and surface enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, G.; Wu, X.; Shi, G. Electrochemical fabrication of two-dimensional palladium nanostructures as substrates for surface enhanced Raman scattering. J. Phys. Chem. B 2006, 110, 24585–24592. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Dolinska, J.; Maiyalagan, T.; Opallo, M. Facile and rapid synthesis of Pd nanodendrites for electrocatalysis and surface-enhanced Raman scattering applications. Nanoscale 2014, 6, 11169–11176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Chong, X.; Chen, Y.; Yang, L.; Luo, L.; Zhao, B.; Tian, Y. Detection of 6-Thioguanine by surface-enhanced Raman scattering spectroscopy using silver nanoparticles-coated silicon wafer. Colloids Surf. A Physicochem. Eng. Asp. 2016, 493, 52–58. [Google Scholar] [CrossRef]
- Ye, W.; Liu, J.; Liu, Q.; Zhou, F.; Liu, W. Surfactant-free and controllable synthesis of hierarchical platinum nanostructures and their comparative studies in electrocatalysis, surface-enhanced Raman scattering and surface wettability. Electrochim. Acta 2010, 55, 8649–8654. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Huang, W.; Yu, L.; Ren, X.; Wen, W.; Yu, S. Ordering Ag nanowire arrays by a glass capillary: A portable, reusable and durable SERS substrate. Sci. Rep. 2012, 2, 987. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; White, I. Inkjet-printed paper-based SERS dipsticks and swabs for trace chemical detection. Analyst 2013, 138, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Tian, L.; Singamaneni, S. Paper-Based SERS Swab for Rapid Trace Detection on Real-World Surfaces. ACS Appl. Mater. Interfaces 2010, 2, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Huang, Q. Rapid fabrication of silver nanoparticle-coated filter paper as SERS substrate for low-abundance molecules detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.; Li, D.; Simon, G.; Garnier, G. Gold Nanoparticle-Paper as a Three-Dimensional Surface Enhanced Raman Scattering Substrate. Langmuir 2012, 28, 8782–8790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, M.; Yu, D.; Yang, L. A novel paper rag as ‘D-SERS’ substrate for detection of pesticide residues at various peels. Talanta 2014, 128, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; White, I. Inkjet Printed Surface Enhanced Raman Spectroscopy Array on Cellulose Paper. Anal. Chem. 2010, 82, 9626–9630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and Adhesive Surface Enhance Raman Scattering Active Tape for Rapid Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Geng, Y.; Bao, Z.; Riaz, S.; Li, H. Silver nanoparticles on cotton swabs for improved surface-enhanced Raman scattering, its application to the detection of carbaryl. Microchim. Acta 2016, 183, 1307–1313. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Z.; Sun, T.; Liu, S.; Wu, X.; Ma, Z.; He, J.; Chen, C. Carbon cloth surface-decorated with silver nanoparticles for surface-enhanced Raman scattering. J. Alloys Compd. 2014, 584, 635–639. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Xia, Y.; Tong, L.; Xu, X.; Ying, Y. Polymer Nanofibers Embedded with Aligned Gold Nanorods: A New Platform for Plasmonic Studies and Optical Sensing. Nano Lett. 2012, 12, 3145–3150. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, Y.; Han, S.; Yan, Y.; Zhou, L.; Chen, W.; Zhou, P.; Chen, X.; Roy, V. Controlled Assembly of Silver Nanoparticles Monolayer on 3D Polymer Nanotubes and their Applications. Small 2014, 10, 4645–4650. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Hu, B.; Yao, Q.; Wang, K.; Yu, S. Large-Scale Synthesis of Flexible Free-Standing SERS Substrates with High Sensitivity: Electrospun PVA Nanofibers Embedded with Controlled Alignment of Silver Nanoparticles. ACS Nano 2009, 3, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Su, F.; Wang, K.; Jiang, W.; Men, X.; Liu, W. Study on the friction and wear properties of carbon fabric composites reinforced with micro- and nano-particles. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2005, 404, 251–258. [Google Scholar] [CrossRef]
- Tran, M.; Mundt, C.; Lan, T.; Padalkar, S. Electrodeposition of Gold Nanostructures Having Controlled Morphology. J. Nanosci. Nanotechnol. 2018, 18, 3492–3498. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Mundt, C.; Tran, M.; Padalkar, S. Effect of gold underlayer on copper(I) oxide photocathode performance. J. Mater. Res. 2017, 32, 1656–1664. [Google Scholar] [CrossRef]
- Tran, M.; DePenning, R.; Turner, M.; Padalkar, S. Effect of Citrate Ratio and Temperature on Gold Nanoparticle Size and Morphology. Mater. Res. Express 2016, 3, 105027. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Zhou, X. Synthesis of silver nanocubes as a SERS substrate for the determination of pesticide paraoxon and thiram. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.; Henry, A.; Ringe, E.; Van Duyne, R. SERS: Materials, applications, the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Doering, W.; Nie, S. Single-molecule and single-nanoparticle SERS: Examining the roles of surface active sites and chemical enhancement. J. Phys. Chem. B 2002, 106, 311–317. [Google Scholar] [CrossRef]
- He, X.; Gao, Y.; Mahjouri-Samani, M.; Black, P.; Allen, J.; Mitchell, M.; Xiong, W.; Zhou, Y.; Jiang, L.; Lu, Y. Surface-enhanced Raman spectroscopy using gold-coated horizontally aligned carbon nanotubes. Nanotechnology 2012, 23, 205702. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hasegawa, K.; Hasegawa, Y.; Takahashi, N.; Kitahama, Y.; Fukuoka, S.; Murase, N.; Baba, Y.; Ozaki, Y.; Itoh, T. Direct conversion of silver complexes to nanoscale hexagonal columns on a copper alloy for plasmonic applications. Phys. Chem. Chem. Phys. 2013, 15, 14611–14615. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, P.; Stockburger, M. Surface-enhanced resonance Raman-spectroscopy of Rhodamine-6G adsorbed on colloidal silver. J. Phys. Chem. 1984, 88, 5935–5944. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Gao, G.; Kong, Y.; Zhi, X.; Wang, K.; Zhang, X.; Cui, D. Biosynthesis of gold nanoparticles using chloroplasts. Int. J. Nanomed. 2011, 6, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Lagugne-Labarthet, F.; Pedersen, D.; Kraatz, H. Studies of the interaction of two organophosphonates with nanostructured silver surfaces. Analyst 2012, 137, 4448–4453. [Google Scholar] [CrossRef] [PubMed]








| Element | C | O | F | Cl | Au | Total |
|---|---|---|---|---|---|---|
| Au/C-Cloth | 2.86 | 0.20 | N/A | 0.03 | 96.91 | 100.00 |
| Au/C-paper | 2.56 | 0.37 | 0.37 | 0.07 | 96.62 | 100.00 |
| Raman Mode (cm−1) | Assignment for R6G | Reference |
|---|---|---|
| 1361 | Aromatic C–C stretching, in-plane C–H bending | [53,54,55,56] |
| 1506, 1532 | Aromatic C–C stretching, C–N stretching, C–H bending, N–H bending | [53,54,55] |
| 1573, 1600 | Aromatic C–C stretching, in-plane N–H bending | [53,54,55] |
| 1650 | Aromatic C–C stretching, in-plane C–H bending | [53,54,55,56] |
| Raman Peak (cm−1) | Assignment for Paraoxon | Reference |
| 732 | NO2 scissor, C–C bending | [3,57] |
| 859 | NO2 scissor (Aromatic–NO2) | [3,57] |
| 1110 | C–H band (in plane)/NO2 asymmetric stretching | [3,57] |
| 1348 | Symmetry stretching NO2 | [3,57] |
| 1592 | Phenyl ring vibration | [3,57] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, M.; Fallatah, A.; Whale, A.; Padalkar, S. Utilization of Inexpensive Carbon-Based Substrates as Platforms for Sensing. Sensors 2018, 18, 2444. https://doi.org/10.3390/s18082444
Tran M, Fallatah A, Whale A, Padalkar S. Utilization of Inexpensive Carbon-Based Substrates as Platforms for Sensing. Sensors. 2018; 18(8):2444. https://doi.org/10.3390/s18082444
Chicago/Turabian StyleTran, Minh, Ahmad Fallatah, Alison Whale, and Sonal Padalkar. 2018. "Utilization of Inexpensive Carbon-Based Substrates as Platforms for Sensing" Sensors 18, no. 8: 2444. https://doi.org/10.3390/s18082444