A Self-Calibrated Non-Parametric Time Series Analysis Approach for Assessing Insect Defoliation of Broad-Leaved Deciduous Nothofagus pumilio Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
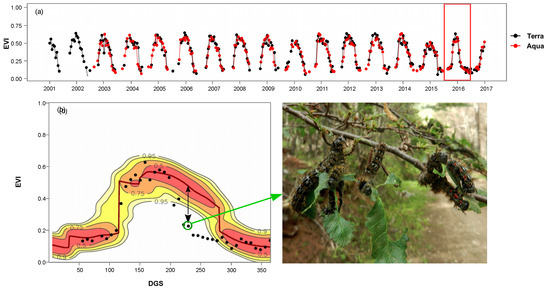
2.2. MODIS Time Series Data and Quality Assessment
2.3. Insect Outbreak Detection and Mapping
2.4. Field Validation and Field Leaf Area Index Data
3. Results
3.1. Calculation of EVI Loss (%) and Anomaly Probability
3.2. Spatiotemporal Patterns of the O. amphimone Outbreak
3.3. Remote Sensing and Field Measurements of Defoliation in the Trapananda National Reserve
4. Discussion
4.1. Performance of the Self-Calibrated Non-Parametric Approach
4.2. Considerations about the VI Time-Series Quality
4.3. Performance of EVI for Detecting LAI Loss Due to Defoliation
4.4. Opportunities for Forest Pest Management
4.5. Potential for Future Forest Insect Outbreak Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances. Bioscience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Fleming, R.A.; Volney, W.J.A. Effects of climate change on insect defoliator population processes in Canada’s boreal forest: Some plausible scenarios. Water. Air Soil Pollut. 1995, 82, 445–454. [Google Scholar] [CrossRef]
- Fraser, R.H.; Latifovic, R. Mapping insect induced tree defoliation and mortality using coarse spatial resolution satellite imagery. Int. J. Remote Sens. 2005, 26, 193–200. [Google Scholar] [CrossRef]
- Axelson, J.N.; Alfaro, R.I.; Hawkes, B.C. Changes in stand structure in uneven-aged lodgepole pine stands impacted by mountain pine beetle epidemics and fires in central British Columbia. For. Chron. 2010, 86, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Berryman, A.A. Forest Insects: Principles and Practice of Population Management; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 1468450808. [Google Scholar]
- Yang, L.H. The Ecological Consequences of Insect Outbreaks. In Insect Outbreaks Revisited; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 197–218. ISBN 9781118295205. [Google Scholar]
- Schowalter, T.D. Outbreaks and Ecosystem Services. In Insect Outbreaks Revisited; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 246–265. [Google Scholar]
- Senf, C.; Seidl, R.; Hostert, P. Remote sensing of forest insect disturbances: Current state and future directions. Int. J. Appl. Earth Obs. Geoinf. 2017, 60, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Rullan-Silva, C.D.; Olthoff, A.E.; Delgado de la Mata, J.A.; Pajares-Alonso, J.A. Remote monitoring of forest insect defoliation. A review. For. Syst. 2013, 22, 377–391. [Google Scholar] [CrossRef]
- Piper, F.I.; Fajardo, A. Foliar habit, tolerance to defoliation and their link to carbon and nitrogen storage. J. Ecol. 2014, 102, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Paritsis, J.; Veblen, T.T.; Smith, J.M.; Holz, A. Spatial prediction of caterpillar (Ormiscodes) defoliation in Patagonian Nothofagus forests. Landsc. Ecol. 2011, 26, 791–803. [Google Scholar] [CrossRef]
- Piper, F.I.; Gundale, M.J.; Fajardo, A. Extreme defoliation reduces tree growth but not C and N storage in a winter-deciduous species. Ann. Bot. 2015, 115, 1093–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, 78–91. [Google Scholar] [CrossRef]
- Lee, K.S.; Cohen, W.B.; Kennedy, R.E.; Maiersperger, T.K.; Gower, S.T. Hyperspectral versus multispectral data for estimating leaf area index in four different biomes. Remote Sens. Environ. 2004, 91, 508–520. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Peterson, D.L. Estimating the foliar biochemical concentration of leaves with reflectance spectrometry: Testing the Kokaly and Clark methodologies. Remote Sens. Environ. 2001, 76, 349–359. [Google Scholar] [CrossRef]
- Coops, N.C.; Waring, R.H.; Wulder, M.A.; White, J.C. Prediction and assessment of bark beetle-induced mortality of lodgepole pine using estimates of stand vigor derived from remotely sensed data. Remote Sens. Environ. 2009, 113, 1058–1066. [Google Scholar] [CrossRef]
- Pontius, J.; Hallett, R.; Martin, M. Using AVIRIS to assess hemlock abundance and early decline in the Catskills, New York. Remote Sens. Environ. 2005, 97, 163–173. [Google Scholar] [CrossRef]
- Wulder, M.A.; Dymond, C.C.; White, J.C.; Leckie, D.G.; Carroll, A.L. Surveying mountain pine beetle damage of forests: A review of remote sensing opportunities. For. Ecol. Manag. 2006, 221, 27–41. [Google Scholar] [CrossRef]
- Spruce, J.P.; Sader, S.; Ryan, R.E.; Smoot, J.; Kuper, P.; Ross, K.; Prados, D.; Russell, J.; Gasser, G.; McKellip, R.; et al. Assessment of MODIS NDVI time series data products for detecting forest defoliation by gypsy moth outbreaks. Remote Sens. Environ. 2011, 115, 427–437. [Google Scholar] [CrossRef]
- Townsend, P.A.; Singh, A.; Foster, J.R.; Rehberg, N.J.; Kingdon, C.C.; Eshleman, K.N.; Seagle, S.W. A general Landsat model to predict canopy defoliation in broadleaf deciduous forests. Remote Sens. Environ. 2012, 119, 255–265. [Google Scholar] [CrossRef]
- Anees, A.; Olivier, J.C.; O’Rielly, M.; Aryal, J. Detecting beetle infestations in pine forests using MODIS NDVI time-series data. In Proceedings of the International Geoscience and Remote Sensing Symposium (IGARSS), Melbourne, Australia, 21–26 July 2013; pp. 3329–3332. [Google Scholar]
- Neigh, S.C.; Bolton, K.D.; Diabate, M.; Williams, J.J.; Carvalhais, N. An Automated Approach to Map the History of Forest Disturbance from Insect Mortality and Harvest with Landsat Time-Series Data. Remote Sens. 2014, 6, 2782–2808. [Google Scholar] [CrossRef] [Green Version]
- Babst, F.; Esper, J.; Parlow, E. Landsat TM/ETM+ and tree-ring based assessment of spatiotemporal patterns of the autumnal moth (Epirrita autumnata) in northernmost Fennoscandia. Remote Sens. Environ. 2010, 114, 637–646. [Google Scholar] [CrossRef]
- Pasquarella, V.J.; Bradley, B.A.; Woodcock, C.E. Near-real-time monitoring of insect defoliation using Landsat time series. Forests 2017, 8, 275. [Google Scholar] [CrossRef]
- Olsson, P.-O.; Lindström, J.; Eklundh, L. Near real-time monitoring of insect induced defoliation in subalpine birch forests with MODIS derived NDVI. Remote Sens. Environ. 2016, 181, 42–53. [Google Scholar] [CrossRef]
- Anees, A.; Aryal, J. Near-real time detection of beetle infestation in pine forests using MODIS data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 3713–3723. [Google Scholar] [CrossRef]
- Eklundh, L.; Johansson, T.; Solberg, S. Mapping insect defoliation in Scots pine with MODIS time-series data. Remote Sens. Environ. 2009, 113, 1566–1573. [Google Scholar] [CrossRef]
- Kleynhans, W.; Olivier, J.C.; Wessels, K.J.; Salmon, B.P.; Van Den Bergh, F.; Steenkamp, K. Detecting land cover change using an extended kalman filter onMODIS NDVI time-series data. IEEE Geosci. Remote Sens. Lett. 2011, 8, 507–511. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Newnham, G.; Culvenor, D. Detecting trend and seasonal changes in satellite image time series. Remote Sens. Environ. 2010, 114, 106–115. [Google Scholar] [CrossRef]
- Broich, M.; Huete, A.; Paget, M.; Ma, X.; Tulbure, M.; Coupe, N.R.; Evans, B.; Beringer, J.; Devadas, R.; Davies, K.; et al. A spatially explicit land surface phenology data product for science, monitoring and natural resources management applications. Environ. Model. Softw. 2015, 64, 191–204. [Google Scholar] [CrossRef]
- Olsson, P.-O.; Kantola, T.; Lyytikäinen-Saarenmaa, P.; Jönsson, A.M.; Eklundh, L. Development of a method for monitoring of insect induced forest defoliation—Limitation of MODIS data in Fennoscandian forest landscapes. Silva Fenn. 2016, 50, 1495. [Google Scholar] [CrossRef]
- Donoso Zegers, C. Las Especies Arbóreas de los Bosques Templados de Chile y Argentina Autoecología; Marisa Cuneo Ediciones: Valdivia, Chile, 2006; ISBN 9567173273. [Google Scholar]
- Veblen, T.T.; Hill, R.S.; Read, J. The Ecology and Biogeography of Nothofagus Forests; Yale University Press: New Haven, CT, USA, 1996; ISBN 0300064233. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. Bioscience 2001, 51, 933. [Google Scholar] [CrossRef]
- Pisano, E. Fitogeografía de Fuego-Patagonia chilena. I.-Comunidades vegetales entre las latitudes 52 y 56° S. In Anales del Instituto de la Patagonia; Revista Universidad de Magallanes: Punta Arenas, Chile, 1977; Volume 8. [Google Scholar]
- Veblen, T.T.; Schlegel, F.M.; Oltremari, J.V. Temperate broad-leaved evergreen forests of South America. In Ecosystems of the World; Ovington, J.D., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1983; pp. 5–31. [Google Scholar]
- CONAF/UACH. Informe Final Estudio “Monitoreo de Cambios, Corrección Cartográfica y Actualización del Catastro de Bosque Nativo en la XI Región de Aisén”. Periodo 1996–2011; Universidad Austral de Chile: Valdivia, Chile, 2012. [Google Scholar]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- White, K.; Pontius, J.; Schaberg, P. Remote sensing of spring phenology in northeastern forests: A comparison of methods, field metrics and sources of uncertainty. Remote Sens. Environ. 2014, 148, 97–107. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- De Beurs, K.M.; Townsend, P.A. Estimating the effect of gypsy moth defoliation using MODIS. Remote Sens. Environ. 2008, 112, 3983–3990. [Google Scholar] [CrossRef]
- Billings, R.F.; Clarke, S.R.; Espino Mendoza, V.; Cordón Cabrera, P.; Meléndez Figueroa, B.; Ramón Campos, J.; Baeza, G. Bark beetle outbreaks and fire: A devastating combination for Central America’s pine forests. Unasylva 2004, 55, 15–21. [Google Scholar]
- Hijmans, R.J.; Van Etten, J. Raster: Geographic Data Analysis and Modeling. R Package Version 2.2-31. 2014. Available online: http//CRAN.R-project.org/package=raster (accessed on 15 March 2014).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project.org/ (accessed on 5 March 2018).
- Didan, K. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid V006; Distributed by NASA EOSDIS LP DAAC; U.S. Geological Survey: Reston, VA, USA, 2015.
- Chávez, R.O.; Estay, S.A.; Riquelme, G. Npphen. An R Package for Estimating Annual Phenological Cycle; Uach, PUCV: Valdivia, Chile, 2017. [Google Scholar]
- Estay, S.A.; Chávez, R.O. Npphen: An R-package for non-parametric reconstruction of vegetation phenology and anomaly detection using remote sensing. bioRxiv 2018, 301143. [Google Scholar] [CrossRef]
- Zimmerman, D.W. A Note on the Influence of Outliers on Parametric and Nonparametric Tests. J. Gen. Psychol. 1994, 121, 391–401. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Fedosejevs, G.; Tiscareño-LóPez, M.; Arnold, J.G. Assessment of MODIS-EVI, MODIS-NDVI and VEGETATION-NDVI Composite Data Using Agricultural Measurements: An Example at Corn Fields in Western Mexico. Environ. Monit. Assess. 2006, 119, 69–82. [Google Scholar] [CrossRef]
- Gu, Y.; Wylie, B.K.; Howard, D.M.; Phuyal, K.P.; Ji, L. NDVI saturation adjustment: A new approach for improving cropland performance estimates in the Greater Platte River Basin, USA. Ecol. Indic. 2013, 30, 1–6. [Google Scholar] [CrossRef]
- Asner, G.P.; Scurlock, J.M.O.; Hicke, J.A. Global synthesis of leaf area index observations: Implications for ecological and remote sensing studies. Glob. Ecol. Biogeogr. 2003, 12, 191–205. [Google Scholar] [CrossRef]
- Chávez, R.O.; Clevers, J.G.P.W.; Herold, M.; Ortiz, M.; Acevedo, E. Modelling the spectral response of the desert tree Prosopis tamarugo to water stress. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 53–65. [Google Scholar] [CrossRef]
- Instituto Nacional De Estadística. Síntesis de Resultados Censo 2017; Istituto Nacional de Estadística: Santiago, Chile, 2017.
- Hall, R.J.; Castilla, G.; White, J.C.; Cooke, B.J.; Skakun, R.S. Remote sensing of forest pest damage: A review and lessons learned from a Canadian perspective. Can. Entomol. 2016, 148, S296–S356. [Google Scholar] [CrossRef]
- Loveland, T.R.; Dwyer, J.L. Landsat: Building a strong future. Remote Sens. Environ. 2012, 122, 22–29. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Ayres, M.P.; Battisti, A.; Larsson, S. Assessing the Impact of Climate Change on Outbreak Potential. In Insect Outbreaks Revisited; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; p. 8. [Google Scholar]
- Olivares-Contreras, V.A.; Mattar, C.; Gutiérrez, A.G.; Jiménez, J.C. Warming trends in Patagonian subantartic forest. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 51–65. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Camarero, J.J.; García-Martín, A.; Hernández, R.; De la Riva, J. Remote-sensing and tree-ring based characterization of forest defoliation and growth loss due to the Mediterranean pine processionary moth. For. Ecol. Manag. 2014, 320, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Sommerfeld, A.; Senf, C.; Buma, B.; D’Amato, A.W.; Després, T.; Díaz-Hormazábal, I.; Fraver, S.; Frelich, L.E.; Gutiérrez, Á.G.; Hart, S.J.; et al. Patterns and drivers of recent disturbances across the temperate forest biome. Nat. Commun. 2018, 9, 4355. [Google Scholar] [CrossRef] [PubMed]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez, R.O.; Rocco, R.; Gutiérrez, Á.G.; Dörner, M.; Estay, S.A. A Self-Calibrated Non-Parametric Time Series Analysis Approach for Assessing Insect Defoliation of Broad-Leaved Deciduous Nothofagus pumilio Forests. Remote Sens. 2019, 11, 204. https://doi.org/10.3390/rs11020204
Chávez RO, Rocco R, Gutiérrez ÁG, Dörner M, Estay SA. A Self-Calibrated Non-Parametric Time Series Analysis Approach for Assessing Insect Defoliation of Broad-Leaved Deciduous Nothofagus pumilio Forests. Remote Sensing. 2019; 11(2):204. https://doi.org/10.3390/rs11020204
Chicago/Turabian StyleChávez, Roberto O., Ronald Rocco, Álvaro G. Gutiérrez, Marcelo Dörner, and Sergio A. Estay. 2019. "A Self-Calibrated Non-Parametric Time Series Analysis Approach for Assessing Insect Defoliation of Broad-Leaved Deciduous Nothofagus pumilio Forests" Remote Sensing 11, no. 2: 204. https://doi.org/10.3390/rs11020204