Hydrothermal Carbonization of Olive Tree Pruning as a Sustainable Way for Improving Biomass Energy Potential: Effect of Reaction Parameters on Fuel Properties
Abstract
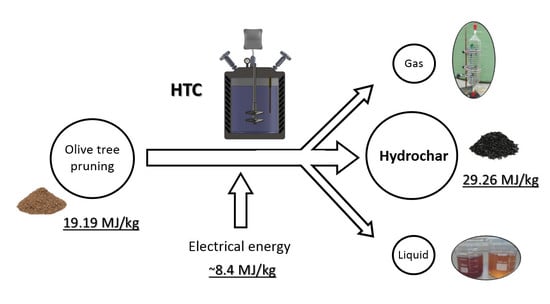
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Experimental Procedure
2.3. Chemical Analysis of the Samples
2.3.1. Solid Phase
2.3.2. Gaseous and Liquid Phases Analysis
2.4. Determination of Combustion Indexes and Activation Energy
2.5. Severity of the Process. Severity Factor
3. Results
3.1. Product Yield
Solid Phase
3.2. Liquid and Gas Phases Characterization
3.3. Chemical Analysis and Higher Heating Value of the Solid Phase
3.4. Thermogravimetric and Fiber Analysis
3.5. Combustion Behavior. Combustion Indexes and Activation Energy
3.6. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

References
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Gani, A.; Morishita, K.; Nishikawa, K.; Naruse, I. Characteristics of Co-combustion of Low-Rank Coal with Biomass. Energy Fuels 2005, 19, 1652–1659. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Chen, Z.; Yoshikawa, K. Clean solid biofuel production from high moisture content waste biomass employing hydrothermal treatment. Appl. Energy 2014, 131, 345–367. [Google Scholar] [CrossRef] [Green Version]
- Martín-Lara, M.A.; Ronda, A.; Zamora, M.C.; Calero, M. Torrefaction of olive tree pruning: Effect of operating conditions on solid product properties. Fuel 2017, 202, 109–117. [Google Scholar] [CrossRef]
- Gao, L.; Volpe, M.; Lucian, M.; Fiori, L.; Goldfarb, J.L. Does hydrothermal carbonization as a biomass pretreatment reduce fuel segregation of coal-biomass blends during oxidation? Energy Convers. Manag. 2019, 181, 93–104. [Google Scholar] [CrossRef]
- Yousaf, B.; Liu, G.; Abbas, Q.; Wang, R.; Ubaid Ali, M.; Ullah, H.; Liu, R.; Zhou, C. Systematic investigation on combustion characteristics and emission-reduction mechanism of potentially toxic elements in biomass- and biochar-coal co-combustion systems. Appl. Energy 2017, 208, 142–157. [Google Scholar] [CrossRef]
- Cuevas, M.; Martínez-Cartas, M.L.; Pérez-Villarejo, L.; Hernández, L.; García-Martín, J.F.; Sánchez, S. Drying kinetics and effective water diffusivities in olive stone and olive-tree pruning. Renew. Energy 2018, 132, 911–920. [Google Scholar] [CrossRef]
- Pérez, A.; Martín-Lara, M.A.; Gálvez-Pérez, A.; Calero, M.; Ronda, A. Kinetic analysis of pyrolysis and combustion of the olive tree pruning by chemical fractionation. Bioresour. Technol. 2018, 249, 557–566. [Google Scholar] [CrossRef]
- Spinelli, R.; Picchi, G. Industrial harvesting of olive tree pruning residue for energy biomass. Bioresour. Technol. 2010, 101, 730–735. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128129920. [Google Scholar]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.W.; Kim, H.J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Liu, F.; Yu, R.; Ji, X.; Guo, M. Hydrothermal carbonization of holocellulose into hydrochar: Structural, chemical characteristics, and combustion behavior. Bioresour. Technol. 2018, 263, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Gallifuoco, A.; Taglieri, L.; Papa, A.A. Hydrothermal carbonization of waste biomass to fuel: A novel technique for analyzing experimental data. Renew. Energy 2020, 149, 1254–1260. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Arenas, C.; Sotres, A.; Alonso, R.M.; González-Arias, J.; Morán, A.; Gómez, X. Pyrolysed almond shells used as electrodes in microbial electrolysis cell. Biomass Convers. Biomass Convers. Biorefin. 2020, 1–9. [Google Scholar] [CrossRef]
- González, J.; Sánchez, M.; Gómez, X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C J. Carbon Res. 2018, 4, 59. [Google Scholar] [CrossRef] [Green Version]
- Toufiq Reza, M.; Freitas, A.; Yang, X.; Hiibel, S.; Lin, H.; Coronella, C.J. Hydrothermal carbonization (HTC) of cow manure: Carbon and nitrogen distributions in HTC products. Environ. Prog. Sustain. Energy 2016, 35, 1002–1011. [Google Scholar] [CrossRef]
- Donar, Y.O.; Çaǧlar, E.; Sinaǧ, A. Preparation and characterization of agricultural waste biomass based hydrochars. Fuel 2016, 183, 366–372. [Google Scholar] [CrossRef]
- Munir, M.T.; Mansouri, S.S.; Udugama, I.A.; Baroutian, S.; Gernaey, K.V.; Young, B.R. Resource recovery from organic solid waste using hydrothermal processing: Opportunities and challenges. Renew. Sustain. Energy Rev. 2018, 96, 64–75. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation. Appl. Energy 2014, 114, 857–864. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A. Upgrading of Olive Tree Trimmings Residue as Biofuel by Hydrothermal Carbonization and Torrefaction: A Comparative Study. In Chemical Engineering Transactions; The Italian Association of Chemical Engineering: Milano, Italy, 2016; Volume 50. [Google Scholar]
- Monedero, E.; Lapuerta, M.; Pazo, A.; Díaz-Robles, L.A.; Pino-Cortés, E.; Campos, V.; Vallejo, F.; Cubillos, F.; Gómez, J. Effect of hydrothermal carbonization on the properties, devolatilization, and combustion kinetics of Chilean biomass residues. Biomass Bioenergy 2019, 130, 105387. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J. Hydrothermal carbonization of loblolly pine: Reaction chemistry and water balance. Biomass Convers. Biorefin. 2014, 4, 311–321. [Google Scholar] [CrossRef]
- He, C.; Tang, C.; Li, C.; Yuan, J.; Tran, K.Q.; Bach, Q.V.; Qiu, R.; Yang, Y. Wet torrefaction of biomass for high quality solid fuel production: A review. Renew. Sustain. Energy Rev. 2018, 91, 259–271. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag. 2016, 47, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- López, R.; Fernández, C.; Cara, J.; Martínez, O.; Sánchez, M.E. Differences between combustion and oxy-combustion of corn and corn-rape blend using thermogravimetric analysis. Fuel Process. Technol. 2014, 128, 376–387. [Google Scholar] [CrossRef]
- Li, X.G.; Ma, B.G.; Xu, L.; Hu, Z.W.; Wang, X.G. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim. Acta 2006, 441, 79–83. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kok, M.V.; Gokalp, I. Thermogravimetric and mass spectrometric (TG-MS) analysis and kinetics of coal-biomass blends. Renew. Energy 2017, 101, 293–300. [Google Scholar] [CrossRef]
- Shafie, S.A.; Al-attab, K.A.; Zainal, Z.A. Effect of hydrothermal and vapothermal carbonization of wet biomass waste on bound moisture removal and combustion characteristics. Appl. Therm. Eng. 2018, 139, 187–195. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. Hydrothermal Carbonization of Waste Biomass for Energy Generation. Procedia Environ. Sci. 2012, 16, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parshetti, G.K.; Kent Hoekman, S.; Balasubramanian, R. Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresour. Technol. 2013, 135, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Zabaniotou, A.; Rovas, D.; Libutti, A.; Monteleone, M. Boosting circular economy and closing the loop in agriculture: Case study of a small-scale pyrolysis-biochar based system integrated in an olive farm in symbiosis with an olive mill. Environ. Dev. 2015, 14, 22–36. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, S.; Hao, N.; Wells, T.; Meng, X.; Li, M.; Pu, Y.; Liu, S.; Ragauskas, A.J. Characterization of products from hydrothermal carbonization of pine. Bioresour. Technol. 2017, 244, 78–83. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Gallifuoco, A.; Taglieri, L.; Scimia, F.; Papa, A.A.; Di Giacomo, G. Hydrothermal carbonization of Biomass: New experimental procedures for improving the industrial Processes. Bioresour. Technol. 2017, 244, 160–165. [Google Scholar] [CrossRef]
- Becker, R.; Dorgerloh, U.; Paulke, E.; Mumme, J.; Nehls, I. Hydrothermal Carbonization of Biomass: Major Organic Components of the Aqueous Phase. Chem. Eng. Technol. 2014, 37, 511–518. [Google Scholar] [CrossRef]
- Basso, D.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of waste biomass: Progress report and prospects. In Proceedings of the 21th European Biomass Conference and Exhibition, Copenhagen, Denmark, 3–7 June 2013. [Google Scholar]
- De la Rubia, M.A.; Villamil, J.A.; Rodriguez, J.J.; Borja, R.; Mohedano, A.F. Mesophilic anaerobic co-digestion of the organic fraction of municipal solid waste with the liquid fraction from hydrothermal carbonization of sewage sludge. Waste Manag. 2018, 76, 315–322. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Unconventional biomass fuels for steam gasification: Kinetic analysis and effect of ash composition on reactivity. Energy 2018, 155, 426–437. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.G.; Shama, G.; Wheatley, A.D.; Sohail, M.; Martin, S.J. Kinetics of faecal biomass hydrothermal carbonisation for hydrochar production. Appl. Energy 2013, 111, 351–357. [Google Scholar] [CrossRef]
- Ozyuguran, A.; Akturk, A.; Yaman, S. Optimal use of condensed parameters of ultimate analysis to predict the calorific value of biomass. Fuel 2018, 214, 640–646. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.; Park, K.Y. Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J. Ind. Eng. Chem. 2016, 42, 95–100. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Mäkelä, M.; Volpe, M.; Volpe, R.; Fiori, L.; Dahl, O. Spatially resolved spectral determination of polysaccharides in hydrothermally carbonized biomass. Green Chem. 2018, 20, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Antal, M.J., Jr.; Allen, S.G.; Dai, X.; Shimizu, B.; Man, S.; Tam, A.; Grønli, M. Attainment of the Theoretical Yield of Carbon from Biomass. Ind. Eng. Chem. Res. 2000, 39, 4024–4031. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Yao, Z. Conversion of sweet potato waste to solid fuel via hydrothermal carbonization. Bioresour. Technol. 2018, 249, 900–907. [Google Scholar] [CrossRef]
- Yang, W.; Wang, H.; Zhang, M.; Zhu, J.; Zhou, J.; Wu, S. Fuel properties and combustion kinetics of hydrochar prepared by hydrothermal carbonization of bamboo. Bioresour. Technol. 2016, 205, 199–204. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Dubey, B.K. Hydrothermal carbonization of yard waste for solid bio-fuel production: Study on combustion kinetic, energy properties, grindability and flowability of hydrochar. Waste Manag. 2019, 91, 108–119. [Google Scholar] [CrossRef]
- Álvarez, A.; Pizarro, C.; García, R.; Bueno, J.L.; Lavín, A.G. Determination of kinetic parameters for biomass combustion. Bioresour. Technol. 2016, 216, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, B.; Chen, C.; Wang, J.; Zhao, M.; Zhang, K. Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour. Technol. 2016, 220, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Elaigwu, S.E.; Greenway, G.M. Microwave-assisted and conventional hydrothermal carbonization of lignocellulosic waste material: Comparison of the chemical and structural properties of the hydrochars. J. Anal. Appl. Pyrolysis 2016, 118, 1–8. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Feng, S.; Wei, R.; Leitch, M.; Xu, C.C. Comparative study on lignocellulose liquefaction in water, ethanol, and water/ethanol mixture: Roles of ethanol and water. Energy 2018, 155, 234–241. [Google Scholar] [CrossRef]
- Lin, L.; Yan, R.; Liu, Y.; Jiang, W. In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicellulose and lignin. Bioresour. Technol. 2010, 101, 8217–8223. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose inbiological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- McCluer, R.H. Methods in carbohydrate chemistry. Volume 3, Cellulose (Whistler, Roy L.; Wolfrom, M.L.; ed.s). J. Chem. Educ. 1964, 41, 352. [Google Scholar] [CrossRef]
- McCluer, R.H. Methods in carbohydrate chemistry. Volume 1, analysis and preparation of sugars (Whistler, Roy, L.; Wolfrom, M.L.; ed.s). J. Chem. Educ. 1963, 40, A394. [Google Scholar] [CrossRef] [Green Version]
- Dence, C.W. The Determination of Lignin. In Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; pp. 33–61. [Google Scholar]
- Rebaque, D.; Martínez-Rubio, R.; Fornalé, S.; García-Angulo, P.; Alonso-Simón, A.; Álvarez, J.M.; Caparros-Ruiz, D.; Acebes, J.L.; Encina, A. Characterization of structural cell wall polysaccharides in cattail (Typha latifolia): Evaluation as potential biofuel feedstock. Carbohydr. Polym. 2017, 175, 679–688. [Google Scholar] [CrossRef] [PubMed]









| Liquid Phase | Gaseous Phase | ||||
|---|---|---|---|---|---|
| Test | pH | TOC (g L−1) | TN (mg L−1) | CO2 (%) | CO (%) |
| 220_3 | 3.7 | 6.3 ± 0.7 | 125 ± 0.9 | 87.6 | 3.7 |
| 220_6 | 3.8 | 6.6 ± 0.2 | 139 ± 8.8 | 92.2 | 2.5 |
| 220_9 | 3.9 | 6.4 ± 0.8 | 109 ± 1.6 | 90.6 | 1.9 |
| 250_3 | 3.8 | 7.5 ± 0.7 | 115 ± 0.5 | 92.6 | 3.8 |
| 250_6 | 3.9 | 6.5 ± 0.4 | 135 ± 12.9 | 92.9 | 3.6 |
| 250_9 | 3.9 | 5.9 ± 0.2 | 116 ± 0.6 | 88.2 | 2.8 |
| 280_3 | 3.8 | 7.5 ± 0.4 | 120 ± 0.3 | 91.0 | 4.0 |
| 280_6 | 3.9 | 6.7 ± 0.8 | 99 ± 0.7 | 91.9 | 2.6 |
| 280_9 | 3.4 | 6.6 ± 0.4 | 143 ± 12.1 | 91.1 | 1.7 |
| Proximate Analysis (%) | Ultimate Analysis (%) | HHV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | M | VM a | FC b | Ash a | C a | H a | N a | O b | S a | (MJ/kg) |
| Feedstock | 31.1 | 85.87 | 12.24 | 1.89 | 48.15 | 5.74 | 0.39 | 45.67 | 0.05 | 19.19 |
| 220_3 | 6.5 | 76.39 | 21.46 | 2.15 | 56.08 | 5.47 | 0.60 | 37.82 | 0.03 | 22.04 |
| 220_6 | 3.5 | 65.90 | 32.04 | 2.06 | 60.22 | 5.34 | 0.76 | 33.63 | 0.05 | 23.89 |
| 220_9 | 3.6 | 60.89 | 35.59 | 3.53 | 61.94 | 5.08 | 0.67 | 32.26 | 0.05 | 24.82 |
| 250_3 | 2.0 | 50.32 | 47.38 | 2.31 | 69.86 | 5.03 | 0.89 | 24.16 | 0.06 | 27.87 |
| 250_6 | 3.4 | 48.05 | 49.90 | 2.05 | 71.67 | 5.05 | 1.13 | 22.09 | 0.06 | 28.73 |
| 250_9 | 4.9 | 47.83 | 50.17 | 2.00 | 73.09 | 5.16 | 1.52 | 20.17 | 0.06 | 29.09 |
| 280_3 | 3.6 | 44.93 | 52.83 | 2.24 | 73.78 | 5.10 | 1.33 | 19.74 | 0.05 | 29.26 |
| 280_6 | 2.9 | 42.33 | 55.48 | 2.18 | 73.86 | 4.88 | 1.18 | 20.04 | 0.04 | 29.09 |
| 280_9 | 3.5 | 40.43 | 57.63 | 1.94 | 75.44 | 4.90 | 1.32 | 18.30 | 0.04 | 29.59 |
| Test | EDR | EY (%) | Ti (°C) | Ci (×10−10) ((%/s)2 °C−3) | FCY (%) | T1 (°C) | Ea 1 (kJ mol−1) | T2 (°C) | Ea 2 (kJ mol−1) |
|---|---|---|---|---|---|---|---|---|---|
| Feedstock | - | - | 255 | 2.0 | - | 326–365 | 21.36 | 378–432 | 18.33 |
| 220_3 | 1.15 | 47.7 | 302 | 3.2 | 8.5 | 286–319 | 93.87 | 495–530 | 91.69 |
| 220_6 | 1.24 | 39.9 | 296 | 2.5 | 10.1 | 279–312 | 84.68 | 487–540 | 81.25 |
| 220_9 | 1.29 | 47.1 | 295 | 1.6 | 12.7 | 270–311 | 59.39 | 488–522 | 156.82 |
| 250_3 | 1.45 | 35.4 | 385 | 1.7 | 11.4 | - | - | 425–469 | 49.81 |
| 250_6 | 1.49 | 33.7 | 403 | 3.3 | 11.1 | - | - | 430–470 | 62.24 |
| 250_9 | 1.52 | 43.5 | 395 | 3.8 | 13.9 | - | - | 424–456 | 54.29 |
| 280_3 | 1.53 | 32.9 | 380 | 3.4 | 11.2 | - | - | 400–438 | 43.88 |
| 280_6 | 1.52 | 31.9 | 413 | 4.8 | 11.5 | - | - | 423–452 | 56.18 |
| 280_9 | 1.54 | 36.5 | 400 | 5.1 | 13.4 | - | - | 400–441 | 52.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Arias, J.; Sánchez, M.E.; Martínez, E.J.; Covalski, C.; Alonso-Simón, A.; González, R.; Cara-Jiménez, J. Hydrothermal Carbonization of Olive Tree Pruning as a Sustainable Way for Improving Biomass Energy Potential: Effect of Reaction Parameters on Fuel Properties. Processes 2020, 8, 1201. https://doi.org/10.3390/pr8101201
González-Arias J, Sánchez ME, Martínez EJ, Covalski C, Alonso-Simón A, González R, Cara-Jiménez J. Hydrothermal Carbonization of Olive Tree Pruning as a Sustainable Way for Improving Biomass Energy Potential: Effect of Reaction Parameters on Fuel Properties. Processes. 2020; 8(10):1201. https://doi.org/10.3390/pr8101201
Chicago/Turabian StyleGonzález-Arias, Judith, Marta Elena Sánchez, Elia Judith Martínez, Camila Covalski, Ana Alonso-Simón, Rubén González, and Jorge Cara-Jiménez. 2020. "Hydrothermal Carbonization of Olive Tree Pruning as a Sustainable Way for Improving Biomass Energy Potential: Effect of Reaction Parameters on Fuel Properties" Processes 8, no. 10: 1201. https://doi.org/10.3390/pr8101201