Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
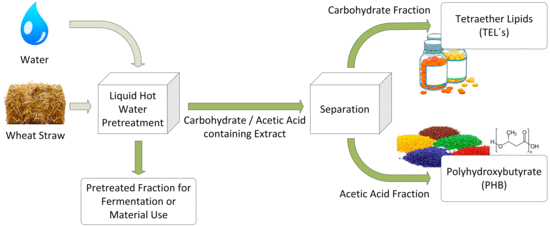
2.2. Process Description
2.3. Wheat Straw Pretreatment
2.4. Extract Separation
2.5. Cultivation of S. acidocaldarius
2.6. Cultivation of Cyanobacteria
2.7. Analytics
3. Results & Discussion
3.1. Wheat Straw Pretreatment
3.2. Fractionation of the Extract
3.3. Cultivation of S. acidocaldarius
3.4. Cultivation of Cyanobacteria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Fan, Y.T.; Xing, Y.; Pan, C.M.; Zhang, G.S.; Lay, J.J. Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenergy 2007, 31, 250–254. [Google Scholar] [CrossRef]
- Cherubini, F.; Strømman, A.H. Chemicals from lignocellulosic biomass: Opportunities, perspectives, and potential of biorefinery systems. Biofuels Bioprod. Biorefining 2011, 5, 548–561. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Liquid Hot Water Pretreatment of Cellulosic Biomass. In Biofuels; Humana Press: Totowa, NJ, USA, 2009; Volume 581, pp. 93–102. [Google Scholar]
- Kamravamanesh, D.; Lackner, M.; Herwig, C. Bioprocess Engineering Aspects of Sustainable Polyhydroxyalkanoate Production in Cyanobacteria. Bioengineering 2018, 5, 111. [Google Scholar] [CrossRef] [Green Version]
- Katayama, N.; Iijima, H.; Osanai, T. Production of Bioplastic Compounds by Genetically Manipulated and Metabolic Engineered Cyanobacteria. In Synthetic Biology of Cyanobacteria; Zhang, W., Song, X., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 155–169. [Google Scholar]
- Quehenberger, J.; Shen, L.; Albers, S.-V.; Siebers, B.; Spadiut, O. Sulfolobus—A Potential Key Organism in Future Biotechnology. Front. Microbiol. 2017, 8, 2474. [Google Scholar] [CrossRef]
- Steinbüchel, A. PHB and Other Polhydroxyalkanoic Acids. In Biotechnology Set; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 403–464. [Google Scholar]
- Liebergesell, M.; Sonomoto, K.; Madkour, M.; Mayer, F.; Steinbüchel, A. Purification and Characterization of the Poly(Hydroxyalkanoic Acid) Synthase from Chromatium vinosum and Localization of the Enzyme at the Surface of Poly(Hydroxyalkanoic Acid) Granules. Eur. J. Biochem. 1994, 226, 71–80. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2012, 24, 803–814. [Google Scholar] [CrossRef]
- Lackner, M. Bioplastics Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 2015. [Google Scholar]
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21. [Google Scholar] [PubMed]
- Grothe, E.; Chisti, Y. Poly(β-hydroxybutyric acid) thermoplastic production by Alcaligenes latus: Behavior of fed-batch cultures. Bioprocess Eng. 2000, 22, 441–449. [Google Scholar] [CrossRef]
- Schubert, P.; Steinbüchel, A.; Schlegel, H.G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J. Bacteriol. 1988, 170, 5837–5847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.F.; Wu, Q.Y.; Shen, Z.Y. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001, 76, 85–90. [Google Scholar] [CrossRef]
- Hänsel, S. Handbook of Climate Change. Mitigation and Adaptation, Second Edition. Environ. Earth Sci. 2018, 77, 554. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Jensen, S.M.; Fricker, G.; Brandl, M.; Treusch, A.H. Archaeal lipids in oral delivery of therapeutic peptides. Eur. J. Pharm. Sci. 2017, 108, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Garg, T.; Rath, G.; Goyal, A.K. Archaeosomes: An excellent carrier for drug and cell delivery. Drug Deliv. 2016, 23, 2497–2512. [Google Scholar] [CrossRef]
- Mahmoud, G.; Jedelská, J.; Omar, S.M.; Strehlow, B.; Schneider, M.; Bakowsky, U. Stabilized tetraether lipids based particles guided prophyrins photodynamic therapy. Drug Deliv. 2018, 25, 1526–1536. [Google Scholar] [CrossRef] [Green Version]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral delivery of vancomycin by tetraether lipid liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef]
- Schiraldi, C.; Marulli, F.; Lernia, I.D.; Martino, A.; De Rosa, M. A microfiltration bioreactor to achieve high cell density in Sulfolobus solfataricus fermentation. Extremophiles 1999, 3, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, J.; Albersmeier, A.; Glatzel, H.; Hackl, M.; Kalinowski, J.; Spadiut, O. A defined cultivation medium for Sulfolobus acidocaldarius. J. Biotechnol. 2019, 301, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Romano, I.; Calandrelli, V.; Pagnotta, E.; Esposito, E.; Di Maso, R. Whey as a medium for biomass production ofSulfolobus solfataricus. Biotechnol. Tech. 1992, 6, 391–392. [Google Scholar] [CrossRef]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 1972, 84, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples. National Renewable Energy Laboratory (NREL): Denver, CO, USA, 2008. [Google Scholar]
- Weinwurm, F.; Cunha, J.; Friedl, A. Pretreatment of wheat straw by liquid hot water and organosolv processes. Chem. Eng. Trans. 2012, 29, 541–546. [Google Scholar]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Gírio, F.M. Wheat straw autohydrolysis: Process optimization and products characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef]
- Ertas, M.; Han, Q.; Jameel, H.; Chang, H. min Enzymatic hydrolysis of autohydrolyzed wheat straw followed by refining to produce fermentable sugars. Bioresour. Technol. 2014, 152, 259–266. [Google Scholar] [CrossRef]
- Han, Q.; Jin, Y.; Jameel, H.; Chang, H.M.; Phillips, R.; Park, S. Autohydrolysis Pretreatment of Waste Wheat Straw for Cellulosic Ethanol Production in a Co-located Straw Pulp Mill. Appl. Biochem. Biotechnol. 2014, 175, 1193–1210. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Vicente, A.A.; Teixeira, J.A. Kinetic modeling of enzymatic saccharification using wheat straw pretreated under autohydrolysis and organosolv process. Ind. Crops Prod. 2012, 36, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Boyer, L.J.; Vega, J.L.; Klasson, K.T.; Clausen, E.C.; Gaddy, J.L. The effects of furfural on ethanol production by saccharomyces cereyisiae in batch culture. Biomass Bioenergy 1992, 3, 41–48. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Palmqvist, E.; Grage, H.; Meinander, N.Q.; Hahn-Hägerdal, B. Main and interaction effects of acetic acid, furfural, andp-hydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnol. Bioeng. 1999, 63, 46–55. [Google Scholar] [CrossRef]
- Grogan, D.W. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J. Bacteriol. 1989, 171, 6710–6719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jaeger, L. Strain Improvement of Oleaginous Microalgae. Ph.D. Thesis, Wageningen University, Wageningen, the Netherlands, 2015; p. 177. [Google Scholar]
- Sharma, L.; Kumar Singh, A.; Panda, B.; Mallick, N. Process optimization for poly-β-hydroxybutyrate production in a nitrogen fixing cyanobacterium, Nostoc muscorum using response surface methodology. Bioresour. Technol. 2007, 98, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.; Jain, P.; Sharma, L.; Mallick, N. Optimization of cultural and nutritional conditions for accumulation of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bioresour. Technol. 2006, 97, 1296–1301. [Google Scholar] [CrossRef]
- Kamravamanesh, D.; Pflügl, S.; Nischkauer, W.; Limbeck, A.; Lackner, M.; Herwig, C. Photosynthetic poly-β-hydroxybutyrate accumulation in unicellular cyanobacterium Synechocystis sp. PCC 6714. AMB Express 2017, 7, 143. [Google Scholar] [CrossRef]






| Arabinan 1 | Galactan 1 | Cellulose 1,2 | Xylane 1 | Mannan 1 | Lignin | Ash |
|---|---|---|---|---|---|---|
| 2.68 | 0.872 | 32.5 | 19.8 | 0.409 | 16.1 | 0.539 |
| ±0.0717 | ±0.0360 | ±0.985 | ±0.432 | ±0.00937 | ±0.518 | ±0.261 |
| Arabinose | Galactose | Glucose | Xylose | Mannose | Acetic Acid | |
|---|---|---|---|---|---|---|
| Monomeric Carbohydrates (g/L) | 0.549 | 0.176 | 5.32 | 0.459 | 0.533 | - |
| Total Carbohydrates (g/L) | 0.833 | 0.953 | 9.03 | 2.17 | 0.732 | - |
| Degradation Products 1 (g/L) | - | - | - | - | - | 1.84 |
| Arabinose | Galactose | Glucose | Xylose | Mannose | Acetic Acid | |
|---|---|---|---|---|---|---|
| Monomeric Carbohydrates (g/L) | 1.22 | 0.090 | 3.67 | 0.0860 | 0.254 | - |
| Total Carbohydrates (g/L) | 2.39 | 2.08 | 11.4 | 2.56 | 0.664 | - |
| Degradation Products 1 (g/L) | - | - | - | - | - | 0.128 |
| mg/L | Arabinose | Galactose | Glucose | Xylose | Mannose | Acetic Acid |
|---|---|---|---|---|---|---|
| Before AC Treatment | 609 | 45 | 1835 | 43 | 127 | 64 |
| After AC Treatment | 167 | 25 | 1534 | - | 41 | 56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beisl, S.; Quehenberger, J.; Kamravamanesh, D.; Spadiut, O.; Friedl, A. Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study. Processes 2019, 7, 956. https://doi.org/10.3390/pr7120956
Beisl S, Quehenberger J, Kamravamanesh D, Spadiut O, Friedl A. Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study. Processes. 2019; 7(12):956. https://doi.org/10.3390/pr7120956
Chicago/Turabian StyleBeisl, Stefan, Julian Quehenberger, Donya Kamravamanesh, Oliver Spadiut, and Anton Friedl. 2019. "Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study" Processes 7, no. 12: 956. https://doi.org/10.3390/pr7120956