Enhancing Thermal Conductivity of Polyvinylidene Fluoride Composites by Carbon Fiber: Length Effect of the Filler
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Size-Down of CFs
2.3. Preparation of PVDF/CF Composites
2.4. Materials Characterization
3. Results and Discussion
3.1. Length Change of CFs before and after Treatment
3.2. Surface Morphology of the PVDF Composite
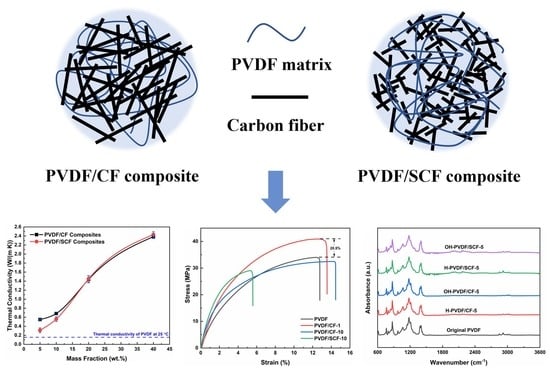
3.3. Thermal Conductivity
3.4. Crystallization Behavior of the Polymer
3.5. Thermal Stability Analysis of PVDF Composites
3.6. Dynamic Mechanical Properties of PVDF Composites
3.7. Mechanical Properties of PVDF Composites
3.8. Chemical Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent advances in polymers and polymer composites for food packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- He, Z.; Mahmud, S.; Yang, Y.; Zhu, L.; Zhao, Y.; Zeng, Q.; Xiong, Z.; Zhao, S. Polyvinylidene fluoride membrane functionalized with zero valent iron for highly efficient degradation of organic contaminants. Sep. Purif. Technol. 2020, 250, 117266. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in Agriculture: A Review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Vatankhah-Varnoosfaderani, M.; Gutiérrez, T.J.; Saeb, M.R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]
- Fu, C.; Yan, C.; Ren, L.; Zeng, X.; Du, G.; Sun, R.; Xu, J.; Wong, C.P. Improving thermal conductivity through welding boron nitride nanosheets onto silver nanowires via silver nanoparticles. Compos. Sci. Technol. 2019, 177, 118–126. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, H.; Liang, C.; Song, P.; Han, Y.; Han, Y.; Gu, J.; Kong, J.; Pan, D.; Guo, Z. Electromagnetic interference shielding MWCNT-Fe3O4@Ag/epoxy nanocomposites with satisfactory thermal conductivity and high thermal stability. Carbon 2018, 141, 506–514. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Luo, X.; Zheng, N.; Ma, T.; Tan, J.; Li, C.; Zhang, Q.; Gu, J. Self-healing, recoverable epoxy elastomers and their composites with desirable thermal conductivities by incorpo-rating BN fillers via in-situ polymerization. Compos. Sci. Technol. 2018, 164, 59–64. [Google Scholar] [CrossRef]
- Li, Y.; Xu, F.; Lin, Z.; Sun, X.; Peng, Q.; Yuan, Y.; Wang, S.; Yang, Z.; He, X.; Li, Y. Electrically and thermally conductive underwater acoustically absorptive graphene/rubber nanocomposites for multifunctional applications. Nanoscale 2017, 9, 14476–14485. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yao, Y.; Gong, Z.; Wang, F.; Sun, R.; Xu, J.; Wong, C.-P. Ice-Templated Assembly Strategy to Construct 3D Boron Nitride Nanosheet Networks in Polymer Composites for Thermal Conductivity Improvement. Small 2015, 11, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Yao, Y.; Zeng, X.; Sun, J.; Hu, J.; Sun, R.; Xu, J.; Wong, C.-P. Learning from Natural Nacre: Constructing Layered Polymer Composites with High Thermal Conductivity. ACS Appl. Mater. Interfaces 2017, 9, 33001–33010. [Google Scholar] [CrossRef] [PubMed]
- Han, U.; Kang, H.; Lim, H.; Han, J.; Lee, H. Development and design optimization of novel polymer heat exchanger using the multi-objective genetic algorithm. Int. J. Heat Mass Transf. 2019, 144, 118589. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ni, H.; Chen, J.; Yang, W. Facile Method to Fabricate Highly Thermally Conductive Graphite/PP Composite with Network Structures. ACS Appl. Mater. Interfaces 2016, 8, 19732–19738. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’Ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Kim, H.; Lohani, P.C.; Bhattarai, D.P.; Tiwari, A.P.; Kim, H.Y. Phytic Acid-Enhanced Electrospun PCL-Polypyrrole Nanofibrous Mat: Preparation, Characterization, and Mechanism. Macromol. Res. 2022, 1–8. [Google Scholar] [CrossRef]
- Ding, D.; Huang, R.; Wang, X.; Zhang, S.; Wu, Y.; Zhang, X.-A.; Qin, G.; Liu, Z.; Zhang, Q.; Chen, Y. Thermally conductive silicone rubber composites with vertically oriented carbon fibers: A new perspective on the heat conduction mechanism. Chem. Eng. J. 2022, 441, 136104. [Google Scholar] [CrossRef]
- Wang, S.; He, H.; Li, Q.; Huang, B.; Wang, H.; Zhang, C. Improving thermal conductivity of ethylene-vinyl acetate composites by covalent bond-connected carbon nano-tubes@boron nitride hybrids. Compos. Commun. 2022, 29, 100986. [Google Scholar] [CrossRef]
- Hou, X.; Chen, Y.; Dai, W.; Wang, Z.; Li, H.; Lin, C.-T.; Nishimura, K.; Jiang, N.; Yu, J. Highly thermal conductive polymer composites via constructing micro-phragmites communis structured carbon fibers. Chem. Eng. J. 2019, 375, 121921. [Google Scholar] [CrossRef]
- Lohani, P.C.; Tiwari, A.P.; Chhetri, K.; Muthurasu, A.; Dahal, B.; Chae, S.-H.; Ko, T.H.; Lee, J.Y.; Chung, Y.S.; Kim, H.Y. Polypyrrole Nanotunnels with Luminal and Abluminal Layered Double Hydroxide Nanosheets Grown on a Carbon Cloth for Energy Storage Applications. ACS Appl. Mater. Interfaces 2022, 14, 23285–23296. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Li, X.; Zhao, S.; Hu, Y.; Zhong, Z.; Xing, W.; Wang, H. Multifunctional metal organic framework and carbon nanotube-modified filter for combined ultrafine dust capture and SO2 dynamic adsorption. Environ. Sci. Nano 2018, 5, 3023–3031. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, Z.; Wang, Z.; Liu, Y.; He, Z.; Cao, A.; Zhou, L.; Zhu, L.; Zhao, S. Super-adsorptive and photo-regenerable carbon nanotube based membrane for highly efficient water purification. J. Membr. Sci. 2020, 621, 119000. [Google Scholar] [CrossRef]
- Yao, S.-S.; Jin, F.-L.; Rhee, K.Y.; Hui, D.; Park, S.-J. Recent advances in carbon-fiber-reinforced thermoplastic composites: A review. Compos. Part B Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Shi, X.; Xu, Y.-J.; Long, J.-W.; Zhao, Q.; Ding, X.-M.; Chen, L.; Wang, Y.-Z. Layer-by-layer assembled flame-retardant architecture toward high-performance carbon fiber composite. Chem. Eng. J. 2018, 353, 550–558. [Google Scholar] [CrossRef]
- Al-Hajaj, Z.; Zdero, R.; Bougherara, H. Mechanical, morphological, and water absorption properties of a new hybrid composite material made from 4 harness satin woven carbon fibres and flax fibres in an epoxy matrix. Compos. Part A Appl. Sci. Manuf. 2018, 115, 46–56. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Liu, D.; Yan, C.; Zhu, Y. A novel and facile fabrication of polyphosphazene nanotube/carbon fiber multi-scale hybrid reinforcement and its enhancing effect on the interfacial properties of epoxy composites. Compos. Sci. Technol. 2018, 169, 34–44. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Wu, G.; Song, G.; Li, X.; Han, P.; Wang, G.; Huang, Y. Interfacial enhancement of carbon fiber composites by growing TiO2 nanowires onto amine-based functionalized carbon fiber surface in supercritical water. Appl. Surf. Sci. 2018, 433, 560–567. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, Y.; Wang, M.; Yang, X.; Song, G.; Huang, Y. Enhancing interfacial strength of epoxy resin composites via evolving hyperbranched amino-terminated POSS on carbon fiber surface. Compos. Sci. Technol. 2018, 170, 148–156. [Google Scholar] [CrossRef]
- Zhao, M.; Meng, L.; Ma, L.; Ma, L.; Yang, X.; Huang, Y.; Ryu, J.E.; Shankar, A.; Li, T.; Yan, C.; et al. Layer-by-layer grafting CNTs onto carbon fibers surface for enhancing the interfacial properties of epoxy resin composites. Compos. Sci. Technol. 2018, 154, 28–36. [Google Scholar] [CrossRef]
- Chen, X.; Su, Y.; Reay, D.; Riffat, S. Recent research developments in polymer heat exchangers—A review. Renew. Sustain. Energy Rev. 2016, 60, 1367–1386. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- He, Z.; Mahmud, S.; Zhao, S.; Yang, Y.; Zhu, L.; Zhao, Y.; Zeng, Q.; Xiong, Z.; Hu, C. Hierarchically Active Poly(vinylidene fluoride) Membrane Fabricated by In Situ Generated Zero-Valent Iron for Fouling Reduction. ACS Appl. Mater. Interfaces 2020, 12, 10993–11004. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; He, Z.; Mahmud, S.; Yang, Y.; Zhou, L.; Hu, C.; Zhao, S. Simple Amphoteric Charge Strategy to Reinforce Superhydrophilic Polyvinylidene Fluoride Membrane for Highly Efficient Separation of Various Surfactant-Stabilized Oil-in-Water Emulsions. ACS Appl. Mater. Interfaces 2020, 12, 47018–47028. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Ullah, H.; Ahmed, I.; Zhan, Y.; Kausar, A.; Aleem, M.A.; Ahmad, S. Investigation of morphology, crystallinity, thermal stability, piezoelectricity and conductivity of PVDF nano-composites reinforced with epoxy functionalized MWCNTs. Compos. Sci. Technol. 2021, 211, 108841. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Q.; Liu, J.; Du, C.; Li, B. Improved interfacial properties for largely enhanced thermal conductivity of poly(vinylidene fluoride)-based nano-composites via functionalized multi-wall carbon nanotubes. Appl. Surf. Sci. 2019, 487, 379–388. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, M.; Liu, Z.; Wu, Y.; Xiong, X.; Li, C.; Wang, X.; Jiang, N.; Yu, J.; Lin, C.-T. Enhanced thermal conductivity for poly(vinylidene fluoride) composites with nano-carbon fillers. RSC Adv. 2016, 6, 68357–68362. [Google Scholar] [CrossRef]
- Guo, H.; Li, X.; Wang, Z.; Li, B.; Wang, J.; Wang, S. Thermal conductivity of PVDF/PANI-nanofiber composite membrane aligned in an electric field. Chin. J. Chem. Eng. 2018, 26, 1213–1218. [Google Scholar] [CrossRef]
- Sanada, K.; Tada, Y.; Shindo, Y. Thermal conductivity of polymer composites with close-packed structure of nano and micro fillers. Compos. Part A Appl. Sci. Manuf. 2009, 40, 724–730. [Google Scholar] [CrossRef]
- Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Thermal conductivity of polymeric composites: A review. In Proceedings of the 2013 IEEE International Conference on Solid Dielectrics (ICSD), Bologna, Italy, 30 June–4 July 2013. [Google Scholar]
- Sobkowicz, M.J.; White, E.A.; Dorgan, J.R. Supramolecular bionanocomposites 3: Effects of surface functionality on electrical and mechanical percolation. J. Appl. Polym. Sci. 2011, 122, 2563–2572. [Google Scholar] [CrossRef]
- Meng, X.; Yu, H.; Wang, L.; Wu, X.; Amin, B.U. Recent Progress on Fabrication and Performance of Polymer Composites with Highly Thermal Conductivity. Macromol. Mater. Eng. 2021, 306, 2100434. [Google Scholar] [CrossRef]
- Ke, K.; Wang, Y.; Zhang, K.; Luo, Y.; Yang, W.; Xie, B.; Yang, M. Melt viscoelasticity, electrical conductivity, and crystallization of PVDF/MWCNT composites: Effect of the dispersion of MWCNTs. J. Appl. Polym. Sci. 2011, 125, E49–E57. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Cui, L.; Wang, J.; Lee, C.; Dong, Y. Simulation study of an open compression absorption heat pump in water and heat recovery of low-temperature and high-humidity flue gas. Energy Convers. Manag. 2022, 269, 116180. [Google Scholar] [CrossRef]
- Huang, F.Y.; Reprogle, R. Thermal Conductivity of Polyvinylidene Fluoride Membranes for Direct Contact Membrane Distillation. Environ. Eng. Sci. 2019, 36, 420–430. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Guthy, C.; Lukes, J.R.; Fischer, J.E.; Winey, K.I. Single Wall Carbon Nanotube/Polyethylene Nanocomposites: Thermal and Electrical Conductivity. Macromolecules 2007, 40, 2417–2421. [Google Scholar] [CrossRef]
- Tonpheng, B.; Yu, J.; Andersson, O. Thermal Conductivity, Heat Capacity, and Cross-Linking of Polyisoprene/Single-Wall Carbon Nanotube Composites under High Pressure. Macromolecules 2009, 42, 9295–9301. [Google Scholar] [CrossRef]
- Yu, J.; Sundqvist, B.; Tonpheng, B.; Andersson, O. Thermal conductivity of highly crystallized polyethylene. Polymer 2014, 55, 195–200. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, O.; Bessard, E.; Bernhart, G. DSC Investigation of the influence of carbon content on peek crystallisation and stability. In Proceedings of the ICCM 19-19th International Conference on Composite Materials, Montreal, QC, Canada, 28 July–2 August 2013. [Google Scholar]
- Lu, F.J.; Hsu, S.L. Study of the crystallization behavior of poly(vinylidene fluoride) from melt under the effect of an electric field. Macromolecules 1986, 19, 326–329. [Google Scholar] [CrossRef]
- Ince-Gunduz, B.S.; Alpern, R.; Amare, D.; Crawford, J.; Dolan, B.; Jones, S.; Kobylarz, R.; Reveley, M.; Cebe, P. Impact of nanosilicates on poly(vinylidene fluoride) crystal polymorphism: Part 1. Melt-crystallization at high supercooling. Polymer 2010, 51, 1485–1493. [Google Scholar] [CrossRef]
- Xiang, F.; Wu, J.; Liu, L.; Huang, T.; Wang, Y.; Chen, C.; Peng, Y.; Jiang, C.; Zhou, Z. Largely enhanced ductility of immiscible high density polyethylene/polyamide 6 blends via nano-bridge effect of functionalized multiwalled carbon nanotubes. Polym. Adv. Technol. 2010, 22, 2533–2542. [Google Scholar] [CrossRef]
- Zhang, W.B.; Xu, X.L.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y.; Zhou, Z.W. High thermal conductivity of poly(vinylidene fluoride)/carbon nanotubes nanocomposites achieved by adding polyvinylpyrrolidone. Compos. Sci. Technol. 2015, 106, 1–8. [Google Scholar] [CrossRef]
- Yu, J.; Qian, R.; Jiang, P. Enhanced thermal conductivity for PVDF composites with a hybrid functionalized graphene sheet-nanodiamond filler. Fibers Polym. 2013, 14, 1317–1323. [Google Scholar] [CrossRef]
- Mokhtari, F.; Spinks, G.M.; Sayyar, S.; Foroughi, J. Dynamic Mechanical and Creep Behaviour of Meltspun PVDF Nanocomposite Fibers. Nanomaterials 2021, 11, 2153. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, H.; Bian, J.; Yan, L.; Guo, Y.; Lin, H.; Lin, H.; Ma, S.; Zhao, X. Facile preparation and properties of polyvinylidene fluoride dielectric nanocomposites via phase morphology control and incorporation of multiwalled carbon nanotubes conductive fillers. J. Appl. Polym. Sci. 2019, 137, 48463. [Google Scholar] [CrossRef]











| Samples | Fillers | Loadings (wt.%) |
|---|---|---|
| PVDF/CF-5 | CFs | 5 |
| PVDF/CF-10 | 10 | |
| PVDF/CF-20 | 20 | |
| PVDF/CF-40 | 40 | |
| PVDF/SCF-5 | SCFs | 5 |
| PVDF/SCF-10 | 10 | |
| PVDF/SCF-20 | 20 | |
| PVDF/SCF-40 | 40 |
| Sample | α-Phase (%) | β-Phase (%) |
|---|---|---|
| PVDF | 65 | 35 |
| PVDF/CF-5 | 61 | 38 |
| PVDF/SCF-5 | 59 | 41 |
| Sample | Weight Loss Temperature (°C) | Residual (%) | ||
|---|---|---|---|---|
| T5% | T10% | Tmax | ||
| PVDF | 390 | 398 | 425 | 33.5 |
| PVDF/CF-5 | 407 | 414 | 432 | 36.5 |
| PVDF/CF-10 | 403 | 412 | 429 | 40.4 |
| PVDF/CF-20 | 393 | 403 | 432 | 43.2 |
| PVDF/CF-40 | 391 | 399 | 396 | 58.4 |
| PVDF/SCF-5 | 414 | 421 | 440 | 36.8 |
| PVDF/SCF-10 | 404 | 412 | 428 | 40.3 |
| PVDF/SCF-20 | 427 | 435 | 450 | 41.6 |
| PVDF/SCF-40 | 424 | 437 | 450 | 54.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, G.; Li, J.; Henderson, L.C.; Lei, W.; Du, L.; Zhao, S. Enhancing Thermal Conductivity of Polyvinylidene Fluoride Composites by Carbon Fiber: Length Effect of the Filler. Polymers 2022, 14, 4599. https://doi.org/10.3390/polym14214599
Yi G, Li J, Henderson LC, Lei W, Du L, Zhao S. Enhancing Thermal Conductivity of Polyvinylidene Fluoride Composites by Carbon Fiber: Length Effect of the Filler. Polymers. 2022; 14(21):4599. https://doi.org/10.3390/polym14214599
Chicago/Turabian StyleYi, Guoqing, Jingliang Li, Luke C. Henderson, Weiwei Lei, Lian Du, and Shuaifei Zhao. 2022. "Enhancing Thermal Conductivity of Polyvinylidene Fluoride Composites by Carbon Fiber: Length Effect of the Filler" Polymers 14, no. 21: 4599. https://doi.org/10.3390/polym14214599