5-Fluorouracil-Loaded Folic-Acid-Fabricated Chitosan Nanoparticles for Site-Targeted Drug Delivery Cargo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
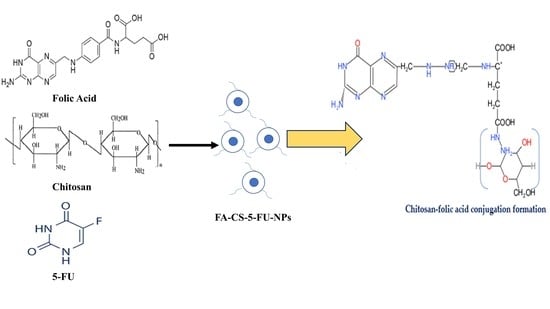
2.2. Conjugation of Folic Acid (FA) with Chitosan (CS)
2.2.1. Fourier Transform Infrared Spectroscopy
2.2.2. H-NMR
2.2.3. Determination of Folate (FA) Content
2.3. Preparation of Nanoparticles (NPs)
2.4. Characterization
2.4.1. NP Size and Zeta Potential
2.4.2. Nanoparticle Morphology
2.4.3. Percentage Yield, Drug Entrapment Efficiency (%EE), and Drug-Loading Efficiency
2.5. In Vitro Release of Nanoparticles
2.6. Cytotoxicity Studies
2.7. Data Analysis and Statistics
3. Results and Discussion
3.1. Synthesis of FA–CS Conjugate
3.1.1. FTIR Studies
3.1.2. H-NMR Study
3.1.3. Folate (FA) Content
3.2. Characterization of Nanoparticles
3.2.1. Size, Zeta Potential, and Surface Morphology
3.2.2. Drug Content, Encapsulation Efficiency, and Drug-Loading Efficiency
3.3. In Vitro Release
3.4. Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, C.S.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jereme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Akhlaq, M.; Azad, A.K.; Ullah, I.; Nawaz, A.; Safdar, M.; Bhattacharya, T.; Uddin, A.B.M.H.; Abbas, S.A.; Mathews, A.; Kundu, S.K.; et al. Methotrexate-Loaded Gelatin and Polyvinyl Alcohol (Gel/PVA) Hydrogel as a pH-Sensitive Matrix. Polymers 2021, 13, 2300. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Dorkoosh, F.A. A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes. Biomed. Pharmacother. 2018, 118, 1259–1273. [Google Scholar] [CrossRef]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and Development of Folic-Acid-Based Receptor Targeting for Imaging and Therapy of Cancer and Inflammatory Diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, Y.K.; Cui, P.; Zhang, J.; Qiao, J.; He, Y.; Jiang, H. Folate-conjugated polyspermine for lung cancer–targeted gene therapy. Acta Pharm. Sin. B 2016, 6, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Akhlaq, M.; Azad, A.K.; Fuloria, S.; Meenakshi, D.U.; Raza, S.; Safdar, M.; Nawaz, A.; Subramaniyan, V.; Sekar, M.; Sathasivam, K.V.; et al. Fabrication of Tizanidine Loaded Patches Using Flaxseed Oil and Coriander Oil as a Penetration Enhancer for Transdermal Delivery. Polymers 2021, 13, 4217. [Google Scholar] [CrossRef]
- Chanphai, P.; Thomas, T.J.; Tajmir-Riahi, H.A. Design of functionalized folic acid–chitosan nanoparticles for delivery of tetracycline, doxorubicin, and tamoxifen. J. Biomol. Struct. Dyn. 2018, 1–7. [Google Scholar] [CrossRef]
- Yang, S.J.; Lin, F.H.; Tsai, H.M.; Lin, C.F.; Chin, H.C.; Wong, J.M.; Shieh, M.J. Alginate-folic acid-modified chitosan nanoparticles for photodynamic detection of intestinal neoplasms. Biomaterials 2011, 32, 2174–2182. [Google Scholar] [CrossRef]
- Gaspar, V.M.; Costa, E.C.; Queiroz, J.A.; Pichon, C.; Sousa, F.; Correia, I.J. Folate-targeted multifunctional amino acid-chitosan nanoparticles for improved cancer therapy. Pharm. Res. 2015, 32, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.K.; Mallick, S.K.; Santra, S.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. In vitro evaluation of folic acid modified carboxymethyl chitosan nanoparticles loaded with doxorubicin for targeted delivery. J. Mater. Sci. Mater. Med. 2010, 2, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Lin, F.H.; Tsai, K.C.; Wei, M.F.; Tsai, H.M.; Wong, J.M.; Shieh, M.J. Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX acolon cancerumulation in colorectal cancer cells. Bioconj. Chem. 2010, 2, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L.; Liu, C.G. Preparation and characterization of self-assemblied nanoparticles based on folic acid modified carboxymethyl chitosan. J. Mat. Sci. Mat. Med. 2011, 22, 1213–1220. [Google Scholar] [CrossRef]
- Malviya, R.; Sundram, S.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Sekar, M.; Kumar, D.H.; Chakravarthi, S.; Porwal, O.; et al. Evaluation and Characterization of Tamarind Gum Polysaccharide: The Biopolymer. Polymers 2021, 13, 3023. [Google Scholar] [CrossRef]
- Khan, T.A.; Azad, A.K.; Fuloria, S.; Nawaz, A.; Subramaniyan, V.; Akhlaq, M.; Fuloria, N.K. Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices. Polymers 2021, 13, 3345. [Google Scholar] [CrossRef]
- Khan, M.A.; Azad, A.K.; Safdar, M.; Nawaz, A.; Akhlaq, M.; Paul, P.; Hossain, M.K.; Rahman, M.H.; Baty, R.S.; El-kott, A.F.; et al. Synthesis and Characterization of Acrylamide/Acrylic Acid Co-Polymers and Glutaraldehyde Crosslinked pH-Sensitive Hydrogels. Gels 2022, 8, 47. [Google Scholar] [CrossRef]
- Bandara, S.; Carnegie, C.A.; Johnson, C.; Akindoju, F.; Williams, E.; Swaby, J.M.; Carson, L.E. Synthesis and characterization of Zinc/Chitosan-Folic acid complex. Heliyon 2018, 4, e00737. [Google Scholar] [CrossRef] [Green Version]
- Musalli, A.H.; Talukdar, P.D.; Roy, P.; Kumar, P.; Wong, T.W. Folate-induced nanostructural changes of oligochitosan nanoparticles and their fate of cellular internalization by melanoma. Carbohydr. Polym. 2020, 244, 116488. [Google Scholar] [CrossRef]
- Akinyelu, J.; Singh, M.J.A.N. Folate-tagged chitosan-functionalized gold nanoparticles for enhanced delivery of 5-fluorouracil to cancer cells. Appl. Nanosci. 2019, 9, 7–17. [Google Scholar] [CrossRef]
- Shah, M.K.A.; Azad, A.K.; Nawaz, A.; Ullah, S.; Latif, M.S.; Rahman, H.; Alsharif, K.F.; Alzahrani, K.J.; El-Kott, A.F.; Albrakati, A.; et al. Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo. Polymers 2022, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A.J.V.S. FT-IR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.H.; Al Omar, S.Y.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Blended Methotrexate-Loaded Transdermal Patches: In Vitro and Ex Vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wu, D.; Liu, L.; Chen, J.; Xu, Y. Preparation, characterization, and in vitro release of folic acid-conjugated chitosan nanoparticles loaded with methotrexate for targeted delivery. Polym. Bull. 2012, 68, 1707–1720. [Google Scholar] [CrossRef]
- Wan, A.; Sun, Y.; Li, H. Characterization of folate-graft-chitosan as a scaffold for nitric oxide release. Int. J. Biol. Macromol. 2008, 43, 415–421. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Jaganathan, S.K.; Ayyar, M.; Krishnasamy, N.P.; Rajasekar, R.; Supriyanto, E. Folic acid decorated chitosan nanoparticles and its derivatives for the delivery of drugs and genes to cancer cells. Curr. Sci. 2017, 1530–1542. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Ince, I.; Yildirim, Y.; Guler, G.; Medine, E.I.; Ballıca, G.; Kusdemir, B.C.; Goker, E. Synthesis and characterization of folic acid-chitosan nanoparticles loaded with thymoquinone to target ovarian cancer cells. J. Radioanal. Nucl. Chem. 2020, 324, 71–85. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Alsaab, H.O.; Sau, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Folic acid conjugated polymeric micelles loaded with a curcumin difluorinated analog for targeting cervical and ovarian cancers. Colloids Surf. B Biointerfaces 2017, 157, 490–502. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Gao, F.; Liu, L.; Zhou, Z.; Zhang, Q. Preparation of folate-modified pullulan acetate nanoparticles for tumor-targeted drug delivery. Drug. Deliv. 2009, 17, 48–57. [Google Scholar] [CrossRef]
- Ramezani Farani, M.; Azarian, M.; Heydari Sheikh Hossein, H.; Abdolvahabi, Z.; Mohammadi Abgarmi, Z.; Moradi, A.; Rabiee, N. Folic acid-adorned curcumin-loaded iron oxide nanoparticles for cervical cancer. ACS Appl. Bio Mater. 2022, 5, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Mattos, A.C.D.; Altmeyer, C.T.; Tania, T.; Khalil, N.M.; Mainardes, R.M. Polymeric nanoparticles for oral delivery of 5-fluorouracil: Formulation optimization, cytotoxicity assay and pre-clinical pharmacokinetics study. Eur. J. Pharm. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]







| Formulation Code | Size (nm) | Zeta Potential (mV) | PDI | Drug Content (%) | Percent Yield | %EE | %LE |
|---|---|---|---|---|---|---|---|
| CS-5-FU NPs | 208 ± 15.00 | +26 ± 2.00 | 0.19 ± 0.01 | 55 ± 1.00 | 90 ± 4.24 | 61 ± 2.00 | 43 ± 3.00 |
| FA-CS-5-FU NPs | 235 ± 12.00 | +20 ± 2.00 | 0.25 ± 0.01 | 53 ± 1.00 | 80.8 ± 3.19 | 59 ± 2.00 | 39 ± 2.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Azad, A.K.; Nawaz, A.; Shah, K.U.; Iqbal, M.; Albadrani, G.M.; Al-Joufi, F.A.; Sayed, A.A.; Abdel-Daim, M.M. 5-Fluorouracil-Loaded Folic-Acid-Fabricated Chitosan Nanoparticles for Site-Targeted Drug Delivery Cargo. Polymers 2022, 14, 2010. https://doi.org/10.3390/polym14102010
Ullah S, Azad AK, Nawaz A, Shah KU, Iqbal M, Albadrani GM, Al-Joufi FA, Sayed AA, Abdel-Daim MM. 5-Fluorouracil-Loaded Folic-Acid-Fabricated Chitosan Nanoparticles for Site-Targeted Drug Delivery Cargo. Polymers. 2022; 14(10):2010. https://doi.org/10.3390/polym14102010
Chicago/Turabian StyleUllah, Shafi, Abul Kalam Azad, Asif Nawaz, Kifayat Ullah Shah, Muhammad Iqbal, Ghadeer M. Albadrani, Fakhria A. Al-Joufi, Amany A. Sayed, and Mohamed M. Abdel-Daim. 2022. "5-Fluorouracil-Loaded Folic-Acid-Fabricated Chitosan Nanoparticles for Site-Targeted Drug Delivery Cargo" Polymers 14, no. 10: 2010. https://doi.org/10.3390/polym14102010