Characterization of the Micelle Formed by a Hydrophobically Modified Pullulan in Aqueous Solution: Size Exclusion Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SEC-MALS
2.3. SAXS
3. Results and Discussion
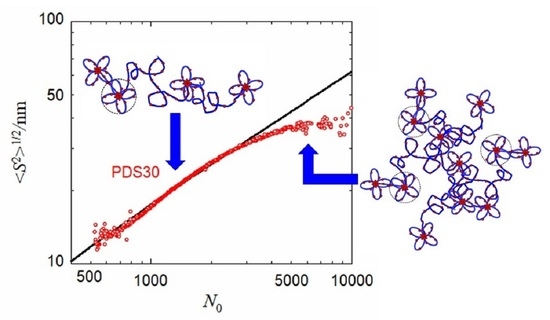
3.1. SEC-MALS
3.2. SAXS
3.3. Fitting of the SAXS Profiles by the Loose Flower Necklace Model
3.4. Comparison of the SEC-MALS Results with the Loose Flower Necklace Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyatt, P.J. Light Scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 1993, 272, l–40. [Google Scholar] [CrossRef]
- Wyatt, P.J. Mean square radius of molecules and secondary instrumental broadening. J. Chromatog. 1993, 648, 27–32. [Google Scholar] [CrossRef]
- Tarazona, M.P.; Saiz, E. Combination of SEC/MALS experimental procedures and theoretical analysis for studying the solution properties of macromolecules. J. Biochem. Biophys. Methods 2003, 56, 95–116. [Google Scholar] [CrossRef]
- Hashidzume, A.; Kawaguchi, A.; Tagawa, A.; Hyoda, K.; Sato, T. Synthesis and Structural Analysis of Self-Associating Amphiphilic Statistical Copolymers in Aqueous Media. Macromolecules 2006, 39, 1135–1143. [Google Scholar] [CrossRef]
- Matsuda, Y.; Biyajima, Y.; Sato, T. Thermal denaturation, renaturation, and aggregation of a double-helical polysaccharide xanthan in aqueous solution. Polym. J. 2009, 41, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Tanohata, D.; Sanada, Y.; Mochizuki, S.; Miyamoto, N.; Sakurai, K. Dilute solution properties of polysaccharide/nucleic acid complexes. Kobunshi Ronbunshu 2015, 72, 37–47. [Google Scholar] [CrossRef]
- Booth, C.; Naylor, T.D.; Price, C.; Rajab, N.S.; Stubberfield, R.B. Investigation of the size distribution of non-ionic micelles formed from a polystyrene-polyisoprene block copolymer in N,N-dimethylacetamide. J. Chem. Soc. Faday Trans. I 1978, 7, 2352–2362. [Google Scholar] [CrossRef]
- Špaček, P.; Kubín, M. Investigation of block copolymer micellization by high-performance size-exclusion chromatography. J. Appl. Polym. Sci. 1985, 30, 143–150. [Google Scholar] [CrossRef]
- Špaček, P. Chromatographic separation of micelle-forming three-block copolymers: Effect of the finite rate of micelle formation on the chromatographic result. J. Appl. Polym. Sci. 1986, 32, 4281–4283. [Google Scholar] [CrossRef]
- Yang, J.; Sato, T. Micellar structure of a hydrophobically modified pullulan in an aqueous solution. Macromolecules 2020, 53, 7970–7979. [Google Scholar] [CrossRef]
- Yang, J.; Sato, T. Transition from the random coil to the flower necklace of a hydrophobically modified pullulan in aqueous solution by changing the degree of substitution. Polymer 2021, 214, 123346. [Google Scholar] [CrossRef]
- Eenschooten, C. Development of Soft Nanocarriers from Novel Amphiphilic Hyaluronic Acid Derivatives towards Drug Delivery. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2008. [Google Scholar]
- Eenschooten, C.; Guillaumie, F.; Kontogeorgis, G.M.; Stenby, E.H.; Schwach-Abdellaoui, K. Preparation and structural characterisation of novel and versatile amphiphilic octenyl succinic anhydride–modified hyaluronic acid derivatives. Carbohydr. Polym. 2010, 79, 597–605. [Google Scholar] [CrossRef]
- Neves-Petersen, M.T.; Klitgaard, S.; Skovsen, E.; Petersen, S.B.; Tømmeraas, K.; Schwach-Abdellaoui, K.J. Biophysical Properties of Phenyl Succinic Acid Derivatised Hyaluronic Acid. J. Fluoresc. 2010, 20, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Tømmeraas, K.; Mellergaard, M.; Malle, B.M.; Skagerlind, P. New amphiphilic hyaluronan derivatives based on modification with alkenyl and aryl succinic anhydrides. Carbohydr. Polym. 2011, 85, 173–179. [Google Scholar] [CrossRef]
- Eenschooten, C.; Vaccaro, A.; Delie, F.; Guillaumie, F.; Tømmeraas, K.; Kontogeorgis, G.M.; Schwach-Abdellaoui, K.; Borkovec, M.; Gurny, R. Novel self-associative and multiphasic nanostructured soft carriers based on amphiphilic hyaluronic acid derivatives. Carbohydr. Polym. 2012, 87, 444–451. [Google Scholar] [CrossRef]
- Ueda, M.; Hashidzume, A.; Sato, T. Unicore-multicore transition of the micelle formed by an amphiphilic alternating copolymer in aqueous media by changing molecular weight. Macromolecules 2011, 44, 2970–2977. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: London, UK, 1992. [Google Scholar]
- Yang, J.; Sato, T. Conformation of pullulan in aqueous solution studied by small-angle X-ray scattering. Polymers 2020, 12, 1266. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Jinbo, Y.; Teramoto, A. Light scattering study of semiflexible polymer solutions II. Application of an integral equation theory. Polym. J. 1995, 27, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Nicolai, T.; Durand, D.; Gimel, J.-C. Static structure factor of dilute solutions of polydlsperse fractal aggregates. Phys. Rev. B 1994, 50, 16357–16363. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Yoshizaki, T. Helical Wormlike Chains in Polymer Solutions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-48714-3. [Google Scholar]
- Kurata, M.; Fukatsu, M. Unperturbed dimension and translational friction constant of branched polymers. J. Chem. Phys. 1964, 41, 2934–2944. [Google Scholar] [CrossRef]






| Sample | DS | M0 | ∂n/∂c/g−1 cm3 |
|---|---|---|---|
| PUL | 0 | 162 | 0.135 |
| PDS14 | 0.14 | 194 | 0.137 |
| PDS20 | 0.20 | 208 | 0.138 |
| PDS30 | 0.30 | 232 | 0.140 |
| PDS42 | 0.42 | 259 | - |
| Sample | DS | N0,w | Ð |
|---|---|---|---|
| PUL | 0 | 1510 | 1.63 |
| PDS14 | 0.14 | 1270 | 1.69 |
| PDS20 | 0.20 | 1200 | 1.69 |
| PDS30 | 0.30 | 1120 | 1.63 |
| Polymer | Flower Micelle Portion | Wormlike Chain Portion | ||||||
|---|---|---|---|---|---|---|---|---|
| dloop/nm a | σ | ∆ρc | h/nm | q/nm | B/nm | db/nm | ||
| PUL | - | - | - | - | 0.35 | 1.5 | 0.3 | 1.0 |
| PUL-OSA | 1.4 | 1.12 | 0.25 | −1.66 | 0.35 | 1.8 | 0.5 | 1.0 |
| Sample | N0 | N0u | nc | nl | qFN/nm | A2b | wagg | Kfractal | α |
|---|---|---|---|---|---|---|---|---|---|
| PDS14 | 1510 | 70 | 1.3 | 20 | 1.8 | 1.6 | 0.01 | 1 × 104 | −2.82 |
| PDS20 | 40 | 15 | 23 | 2.0 | 0.008 | −2.66 | |||
| PDS30 | 30 | 25 | 25 | 2.0 | 0.0085 | −2.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Sato, T. Characterization of the Micelle Formed by a Hydrophobically Modified Pullulan in Aqueous Solution: Size Exclusion Chromatography. Polymers 2021, 13, 1237. https://doi.org/10.3390/polym13081237
Yang J, Sato T. Characterization of the Micelle Formed by a Hydrophobically Modified Pullulan in Aqueous Solution: Size Exclusion Chromatography. Polymers. 2021; 13(8):1237. https://doi.org/10.3390/polym13081237
Chicago/Turabian StyleYang, Jia, and Takahiro Sato. 2021. "Characterization of the Micelle Formed by a Hydrophobically Modified Pullulan in Aqueous Solution: Size Exclusion Chromatography" Polymers 13, no. 8: 1237. https://doi.org/10.3390/polym13081237