Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties
Abstract
:1. Introduction and Historical Background
2. Structural Features and Physical Principles of Antiplasticization
2.1. Molecular and Morphological Considerations
2.1.1. Solubility Parameters as a Criterion for Plasticizer Miscibility
2.1.2. Polymer/Plasticizer Interactions by Fourier Transform Infrared Spectroscopy (FTIR)
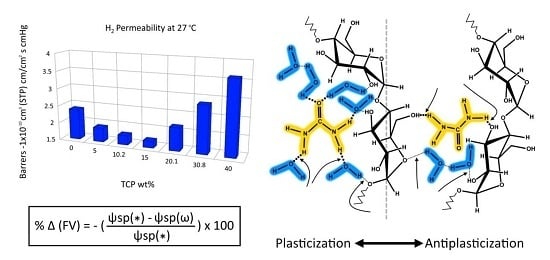
2.1.3. Plasticizer Clustering
2.2. Free Volumes in Relation to Plasticization and Antiplasticization
2.2.1. The Additivity Rule, Free Volume and “Holes”
2.2.2. Deviations from the Additivity Rule
2.2.3. Comparison of Antiplasticization with Physical Ageing
2.2.4. Molecular Weight Considerations
2.3. Molecular Dynamics of Antiplasticization
2.3.1. Relaxations Interpretation from Mechanical and Dielectric Spectra
2.3.2. Melt Fragility as a Parameter for Antiplasticization
3. Implications of Antiplasticization for Applications
3.1. Mechanical Properties
3.1.1. Modulus Enhancement through Antiplasticization
Experimental Data
Modelling Prediction of Elastic Constants
3.1.2. Evaluation of Data from Strength Measurements
3.1.3. Fracture Toughness and Related Phenomena
3.2. Permeation and Diffusion Related Properties
3.2.1. Gas Permeation Barrier and Membranes
3.2.2. Sorption/Desorption and Stability Aspects
3.2.3. The Role of Water in Antiplasticization
4. Peculiarities in the Interpretation of Antiplasticization Phenomena
4.1. The Glass Transition Temperature Anomalies
4.2. Implications of Data on Molecular Glasses
4.3. Antiplasticization in Heterogeneous Polymer Materials
4.3.1. Polymer Blends
4.3.2. Polymer Matrix Composites and Nanocomposites
4.4. Unusual Feature of Antiplasticization
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mascia, L.; Acierno, D. Polymers in industry: From guncotton to CO2 glass. Adv. Polym. Technol. 2012, 31, 179–192. [Google Scholar] [CrossRef]
- Nichols, J.B. Nitrocellulose and Camphor. J. Phys. Chem. 1923, 28, 769–771. [Google Scholar] [CrossRef]
- Brous, S.L.; Semon, W.L. Koroseal A New Plastic Some Properties and Uses. Ind. Eng. Chem. 1935, 27, 667–672. [Google Scholar] [CrossRef]
- Kinjo, N.; Nakagawa, T. Antiplasticization in the Slightly Plasticized Polyvinyl Chloride. J. Soc. Mater. Sci. Jpn. 1973, 22, 462–465. [Google Scholar] [CrossRef]
- Robeson, L.M.; Faucher, J.A. Secondary loss transitions in antiplasticized polymers. J. Polym. Sci. Part B Polym. Lett. 1969, 7, 35–40. [Google Scholar] [CrossRef]
- Kapur, S.; Rogers, C.E.; Baer, E. A mechanism for the β relaxation of wet nylon 6. J. Polym. Sci. Part B Polym. Phys. 1972, 10, 2297–2300. [Google Scholar] [CrossRef]
- Dlubek, G.; Redmann, F.; Krause-Rehberg, R. Humidity-induced plasticization and antiplasticization of polyamide 6: A positron lifetime study of the local free volume. J. Appl. Polym. Sci. 2002, 84, 244–255. [Google Scholar] [CrossRef]
- Serpe, G.; Chaupart, N. Relaxation-structure relationship in bulk and plasticized polyamide 11. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2351–2365. [Google Scholar] [CrossRef]
- Mascia, L. Antiplasticization of poly (vinyl chloride) in relation to thermal ageing and non-linear viscoelastic behaviour. Polymer (Guildf) 1978, 19, 325–328. [Google Scholar] [CrossRef]
- Mascia, L. The influence of deformation mode on the dynamic mechanical spectra of lightly plasticised PVC compositions. Polym. Test. 1987, 7, 109–120. [Google Scholar] [CrossRef]
- Robertson, R.E.; Joynson, C.W. Free volume and the annealing and antiplasticizing of bisphenol A polycarbonate. J. Appl. Polym. Sci. 1972, 16, 733–738. [Google Scholar] [CrossRef]
- Mascia, L.; Margetts, G. Viscoelasticity and plasticity aspects of antiplasticization phenomena: Strain rate and temperature effects. J. Macromol. Sci. Part B. 1987, 26, 237–256. [Google Scholar] [CrossRef]
- Soong, S.Y.; Cohen, R.E.; Boyce, M.C.; Chen, W. The effects of thermomechanical history and strain rate on antiplasticization of PVC. Polymer (Guildf) 2008, 49, 1440–1443. [Google Scholar] [CrossRef]
- Tsui, N.T.; Yang, Y.; Mulliken, A.D.; Torun, L.; Boyce, M.C.; Swager, T.M.; Thomas, E.L. Enhancement to the rate-dependent mechanical behavior of polycarbonate by incorporation of triptycenes. Polymer (Guildf) 2008, 49, 4703–4712. [Google Scholar] [CrossRef]
- Mascia, L.; Wooldridge, P.G.; Stokell, M.J. Antiplasticization of polyvinyl chloride in relation to crazing and fracture behaviour. J. Mater. Sci. 1989, 24, 2775–2780. [Google Scholar] [CrossRef]
- Borek, J.; Osoba, W. Influence of the plasticization on free volume in polyvinyl chloride. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1839–1845. [Google Scholar] [CrossRef]
- Sefcik, M.D.; Schaefer, J.; May, F.L.; Raucher, D.; Dub, S.M. Diffusivity of gases and main-chain cooperative motions in plasticized poly(vinyl chloride). J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 1041–1054. [Google Scholar] [CrossRef]
- Guerrero, S.J. Antiplasticization and crystallinity in poly(vinyl chloride). Macromolecules 1989, 22, 3480–3485. [Google Scholar] [CrossRef]
- Kazama, Y.; Yamamoto, O. A Study on the Antiplasticization Mechanism of PVC by Infrared Dichroism. J. Soc. Mater. Sci. Jpn. 1971, 20, 665–668. [Google Scholar] [CrossRef] [Green Version]
- Delcambre, S.P.; Riggleman, R.A.; de Pablo, J.J.; Nealey, P.F. Mechanical properties of antiplasticized polymer nanostructures. Soft Matter 2010, 6, 2475. [Google Scholar] [CrossRef]
- Cais, R.E.; Nozomi, M.; Kawai, M.; Miyake, A. Antiplasticization and abrasion resistance of polycarbonates in the charge-transport layer of an organic photoconductor. Macromolecules 1992, 25, 4588–4596. [Google Scholar] [CrossRef]
- Ubbink, J. Plasticization and antiplasticization in amorphous food systems. Curr. Opin. Food Sci. 2018, 21, 72–78. [Google Scholar]
- Seow, C.C.; Cheah, P.B.; Chang, Y.P. Antiplasticization by Water in Reduced-Moisture Food Systems. J. Food Sci. 1999, 64, 576–581. [Google Scholar] [CrossRef]
- Pittia, P.; Sacchetti, G. Antiplasticization effect of water in amorphous foods. A review. Food Chem. 2008, 106, 1417–1427. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Moraru, C.I.; Lee, T.-C.; Karwe, M.V.; Kokini, J.L. Plasticizing and Antiplasticizing Effects of Water and Polyols on a Meat-Starch Extruded Matri. J. Food Sci. 2002, 67, 3396–3401. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; León, A.E. Properties of triticale protein films and their relation to plasticizing—Antiplasticizing effects of glycerol and sorbitol. Ind. Crops Prod. 2013, 50, 297–303. [Google Scholar] [CrossRef]
- Lee, J.S.; Leisen, J.; Choudhury, R.P.; Kriegel, R.M.; Beckham, H.W.; Koros, W.J. Antiplasticization-based enhancement of poly(ethylene terephthalate) barrier properties. Polymer (Guildf) 2012, 53, 213–222. [Google Scholar] [CrossRef]
- Klinger, M.; Tolbod, L.P.; Ogilby, P.R. Influence of a novel castor-oil-derived additive on the mechanical properties and oxygen diffusivity of polystyrene. J. Appl. Polym. Sci. 2012, 118, 1643–1650. [Google Scholar] [CrossRef]
- Larocca, N.M.; Pessan, L.A. Effect of antiplasticisation on the volumetric, gas sorption and transport properties of polyetherimide. J. Membr. Sci. 2003, 218, 69–92. [Google Scholar] [CrossRef]
- Ambrosio-Martín, J.; Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. An effect of lactic acid oligomers on the barrier properties of polylactide. J. Mater. Sci. 2014, 49, 2975–2986. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Johansson, C. Mechanical and barrier properties of starch-based films plasticized with two- or three component deep eutectic solvents. Carbohydr. Polym. 2016, 151, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, F.; Zhu, P. Structure and properties of urea-plasticized starch films with different urea contents. Carbohydr. Polym. 2014, 101, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.S.; Douglas, J.F.; Freed, K.F. Influence of Cohesive Energy on the Thermodynamic Properties of a Model Glass-Forming Polymer Melt. Macromolecules 2016, 49, 8341–8354. [Google Scholar] [CrossRef]
- Mascia, L. The Role of Additives in Plastics; Edward Arnold: London, UK, 1974; pp. 58–59. [Google Scholar]
- Bao, C.Y.; Long, D.R.; Vergelati, C. Miscibility and dynamical properties of cellulose acetate/plasticizer systems. Carbohydr. Polym. 2015, 116, 95–102. [Google Scholar] [CrossRef]
- Younker, J.M.; Poladi, R.H.; Bendler, H.V.; Sunkara, H.B. Computational screening of renewably sourced polyalkylene glycol plasticizers for nylon polyamides. Polym. Adv. Technol. 2016, 27, 273–280. [Google Scholar] [CrossRef]
- He, M.; Wang, Z.; Wang, R.; Zhang, L.; Jia, Q. Preparation of Bio-Based Polyamide Elastomer by Using Green Plasticizers. Polymers (Basel) 2016, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Paris, C.; Coupry, C. Fourier transform Raman spectroscopic study of the first cellulose-based artificial materials in heritage. J. Raman Spectrosc. 2005, 36, 77–82. [Google Scholar] [CrossRef]
- Benazzouz, A.; Dudognon, E.; Correia, N.T.; Molinier, V.; Aubry, J.-M.; Descamps, M. Interactions underpinning the plasticization of a polymer matrix: A dynamic and structural analysis of DMP-plasticized cellulose acetate. Cellulose 2017, 24, 487–503. [Google Scholar] [CrossRef]
- De Groote, P.; Rouxhet, P.G.; Devaux, J.; Godard, P. Infrared Study of the Hydrogen Bonding Association in Polyamides Plasticized by Benzenesulfonamides. Part I: Self-Association in Amide and Sulfonamide Systems; Part II: Amide—Sulfonamide Interaction. Appl. Spectrosc. 2001, 55, 877–887. [Google Scholar] [CrossRef]
- Musto, P.; Ragosta, G.; Mascia, L. Vibrational Spectroscopy Evidence for the Dual Nature of Water Sorbed into Epoxy Resins. Chem. Mater. 2000, 12, 1331–1341. [Google Scholar] [CrossRef]
- Bergquist, P.; Zhu, Y.; Jones, A.A.; Inglefield, P.T. Plasticization and Antiplasticization in Polycarbonates: The Role of Diluent Motion. Macromolecules 1999, 32, 7925–7931. [Google Scholar] [CrossRef]
- Ruiz-Treviño, F.A.; Paul, D.R. A quantitative model for the specific volume of polymer-diluent mixtures in the glassy state. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1037–1050. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, G.; Grover, C.P. Volume relaxation in polymers and its effect on waveguide applications. Appl. Opt. 2004, 43, 2325. [Google Scholar] [CrossRef]
- Ronald, P.; White, J.; Lipson, E.G. Polymer Free Volume and its Connection to the Glass Transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar]
- Vrentas, J.S.; Duda, J.L.; Ling, H.C. Antiplasticization and volumetric behavior in glassy polymers. Macromolecules 1988, 21, 1470–1475. [Google Scholar] [CrossRef]
- Sharma, S.K.; Pujari, P.K. Role of free volume characteristics of polymer matrix in bulk physical properties of polymer nanocomposites: A review of positron annihilation lifetime studies. Prog. Polym. Sci. 2017, 75, 31–47. [Google Scholar] [CrossRef]
- Queiroz, S.M.; Machado, J.C.; Porto, A.O.; Silva, G.G. Positron annihilation and differential scanning calorimetry studies of plasticized poly(ethylene oxide). Polymer (Guildf) 2001, 42, 3095–3101. [Google Scholar] [CrossRef]
- Meiers, R.J.; Coussens, B. The molecular structure of the urea molecule: Is the minimum energy structure planar? J. Mol. Struct. Theochem. 1992, 253, 25–33. [Google Scholar] [CrossRef]
- Jackson, W.J.; Caldwell, J.R. Antiplasticization. II. Characteristics of antiplasticizers. J. Appl. Polym. Sci. 1967, 11, 211–226. [Google Scholar] [CrossRef]
- Anderson, S.L.; Grulke, E.A.; DeLassus, P.T.; Smith, P.B.; Kocher, C.W.; Landes, B.G. A Model for Antiplasticization in Polystyrene. Macromolecules 1995, 28, 2944–2954. [Google Scholar] [CrossRef]
- Mascia, L. Thermoplastics Materials Engineering, 2nd ed.; Elsevier Applied Science: London, UK, 1989; p. 62. [Google Scholar]
- Jenckel, E.; Heusch, R. Die Erniedrigung der Einfriertemperatur organischer Gläser durch Lösungsmittel, (Lowering of the glass transition temperature of organic glasses by solvents). Kolloid Z. 1953, 130, 89–105. [Google Scholar] [CrossRef]
- Maeda, Y.; Paul, D.R. Effect of antiplasticization on gas sorption and transport. II. Poly(phenylene oxide). J. Polym. Sci. Part B Polym. Phys. 1987, 25, 981–1003. [Google Scholar] [CrossRef]
- Goudeau, S.; Charlot, M.; Vergelati, C.; Müller-Plathe, F. Atomistic Simulation of the Water Influence on the Local Structure of Polyamide 6,6. Macromolecules 2004, 37, 8072–8081. [Google Scholar] [CrossRef]
- Lamm, M.S.; Simpson, A.; McNevin, M.; Frankenfeld, C.; Nay, R.; Variankaval, N. Probing the Effect of Drug Loading and Humidity on the Mechanical Properties of Solid Dispersions with Nanoindentation: Antiplasticization of a Polymer by a Drug Molecule. Mol. Pharm. 2012, 9, 3396–3402. [Google Scholar] [CrossRef]
- Lee, J.S. Fundamentals of Transport in Advanced Barrier Materials Based on Engineered Antiplasticization. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, May 2011. [Google Scholar]
- Lee, H.-D.; McGarry, F.J. The origin of the β transition and its influence on physical ageing. Polymer 1993, 34, 4267–4271. [Google Scholar] [CrossRef]
- Ito, K.; Ujira, Y. Positronium Diffusion in Polystyrene at Low Temperatures. Polym. J. 1998, 30, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Yavari, M.; Maruf, S.; Lin, H. Physical aging of glassy perfluoropolymers in thin film composite membranes. Part II. Glass transition temperature and the free volume model. J. Membr. Sci. 2017, 525, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Maeda, Y.; Paul, D.R. Effect of antiplasticization on gas sorption and transport. III. Free volume interpretation. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1005–1016. [Google Scholar] [CrossRef]
- Puosi, F.; Leporini, D. The kinetic fragility of liquids as manifestation of the elastic softening. Eur. Phys. J. E 2015, 38, 87. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.J.; Sellaiyan, S.; Kakizaki, T.; Uedono, A.; Taniguchi, Y.; Hayashi, K. Effect of Free-Volume Holes on Dynamic Mechanical Properties of Epoxy Resins for Carbon-Fiber-Reinforced Polymers. Macromolecules 2017, 50, 3933–3942. [Google Scholar] [CrossRef]
- Miyagawa, A.; Nobukawa, S.; Yamaguchi, M. Thermal Expansion Behavior of Antiplasticized Polycarbonate. Nihon Reoroji Gakkaishi 2014, 42, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Chung, T.-S.; Paul, D.R. Physical aging and carbon dioxide plasticization of thin polyimide films in mixed gas permeation. J. Membr. Sci. 2014, 450, 457–468. [Google Scholar] [CrossRef]
- Benczédi, D.; Tomka, I.; Escher, F. Thermodynamics of Amorphous Starch−Water Systems. 1. Volume Fluctuations. Macromolecules 1998, 31, 3055–3061. [Google Scholar] [CrossRef]
- Benczédi, D.; Tomka, I.; Escher, F. Thermodynamics of Amorphous Starch−Water Systems. 2. Concentration Fluctuations. Macromolecules 1998, 31, 3062–3074. [Google Scholar] [CrossRef]
- Dudowicz, J.; Freed, K.; Douglas, J.F. Direct computation of characteristic temperatures and relaxation times for glass-forming polymer liquids. J. Chem. Phys. 2005, 123, 111102. [Google Scholar] [CrossRef]
- Roussenova, M.; Murith, M.; Alam, A.; Ubbink, J. Plasticization, Antiplasticization, and Molecular Packing in Amorphous Carbohydrate-Glycerol Matrices. Biomacromolecules 2010, 11, 3237–3247. [Google Scholar] [CrossRef]
- Araujo, S.; Delpouve, N.; Dhotel, A.; Domenek, S.; Guinault, A.; Delbreilh, L.; Dargent, E. Reducing the Gap between the Activation Energy Measured in the Liquid and the Glassy States by Adding a Plasticizer to Polylactide. ACS Omega 2018, 3, 17092–17099. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Inoue, Y. Enthalpy Relaxation and Embrittlement of Poly(l-lactide) during Physical Aging. Macromolecules 2007, 40, 9664–9671. [Google Scholar] [CrossRef]
- Vilics, T.; Schneider, H.A.; Manoviciu, V.; Manoviciu, L. A DMA Study of the Suppression of the β Transition in slightly Plasticized PVC Blends. J. Therm. Anal. 1996, 47, 1141–1153. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.H.; Cho, M.S.; Choi, S.H.; Lee, Y.K.; Nam, J.D. Viscoelastic characteristics of plasticized cellulose nanocomposites. J. Polym. Sci. Part B: Polym. Phys. 2005, 43, 59–65. [Google Scholar] [CrossRef]
- Seymour, R.W.; Weinhold, S.; Haynes, S.K. Mechanical and dielectric relaxation in cellulose esters. J. Macromol. Sci. Part B. 1979, 16, 337–353. [Google Scholar] [CrossRef]
- Lourdin, D.; Bizot, H.; Colonna, P. Antiplasticization in starch-glycerol films? J. Appl. Polym. Sci. 1997, 63, 1047–1053. [Google Scholar] [CrossRef]
- Psurek, T.; Soles, C.L.; Page, K.A.; Cicerone, M.T.; Douglas, J.F. Quantifying Changes in the High-Frequency Dynamics of Mixtures by Dielectric Spectroscopy. J. Phys. Chem. B 2008, 112, 15980–15990. [Google Scholar] [CrossRef]
- Cangialosi, D.; Wubbenhorst, M.; Schut, H.; van Veen, A.; Picken, S.J. Dynamics of polycarbonate far below the glass transition temperature: A positron annihilation lifetime study. Phys. Rev. B 2004, 69, 134206. [Google Scholar] [CrossRef] [Green Version]
- Elicegui, A.; del Val, J.J.; Bellenger, V.; Verdu, J. A study of plasticization effects in poly(vinyl chloride). Polymer 1997, 38, 1647–1657. [Google Scholar] [CrossRef]
- Maeda, M.; Nobukawa, S.; Inomata, K.; Yamaguchi, M. Effect of molecular size on correlated dynamics of low-mass molecules and local chain motion in antiplasticized polycarbonate. Nihon Reoroji Gakshaishi (J. Soc. Rheol. Jpn.) 2019, 47, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Angell, C. Relaxation in liquids, polymers and plastic crystals - strong/fragile patterns and problems. J. Non-Cryst. Solids 1991, 13, 131–133. [Google Scholar] [CrossRef]
- Floudas, G.; Paluch, M.; Grzybowski, A.; Ngai, K.L. Molecular Dynamics of Glass-Forming Systems; Springer: Berlin/Heidelberg, Germany, 2001; p. 6. [Google Scholar]
- Doolittle, A.K.; Doolittle, D.B. Studies in Newtonian flow. V. Further verification of the free-space viscosity equation. J. Appl. Phys. 1957, 28, 901. [Google Scholar] [CrossRef]
- Stukalin, E.B.; Douglas, J.F.; Freed, K.F. Plasticization and antiplasticization of polymer melts diluted by low molar mass species. J. Chem. Phys. 2010, 132, 084504. [Google Scholar] [CrossRef]
- Riggleman, R.A.; Douglas, J.F.; de Pablo, J.J. Antiplasticization and the elastic properties of glass-forming polymer liquids. Soft Matter 2010, 6, 292–304. [Google Scholar] [CrossRef]
- Garton, A.; Haldankar, G.S.; McLean, P.D. Modification of Free Volume in Epoxy Adhesive Formulations on tests carried out on an epoxy resin. J. Adhes. 1989, 29, 13–26. [Google Scholar] [CrossRef]
- Mikus, P.-Y.; Alix, S.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P.; Coqueret, X.; Dolea, P. Deformation mechanisms of plasticized starch materials. Carbohydr. Polym. 2014, 114, 450–457. [Google Scholar] [CrossRef]
- Oxborough, R.J.; Bowde, P.B. A critical strain criterion for crazing of glassy polymers. Philos. Mag. 1973, 28, 547–559. [Google Scholar] [CrossRef]
- Sternstein, S.S.; Myers, F.A. Solid State of Polymers; Geil, P.H., Baer, E., Wada, Y., Eds.; Marcel Dekker: New York, NY, USA, 1974; p. 54. [Google Scholar]
- Dieter, G.E. Mechanical Metallurgy, 2nd ed.; McGraw Hill: Tokyo, Japan, 1976; p. 681. [Google Scholar]
- Plati, E.; Williams, J.G. Effects of temperature on fracture toughness of polymers. Polymer 1975, 16, 915–920. [Google Scholar] [CrossRef]
- Fujita, H. Diffusion in polymer-diluent systems. In Fortschritte Der Hochpolym; Springer: Berlin/Heidelberg, Germany, 1961; pp. 1–47. [Google Scholar]
- Cohen, M.H.; Turnbull, D. Molecular Transport in Liquids and Glasses. J. Chem. Phys. 1959, 31, 1164–1169. [Google Scholar] [CrossRef] [Green Version]
- Duda, J.L.; Romdhane, I.H.; Danner, R.P. Diffusion in glassy polymers—relaxation and antiplasticization. J. Non-crystalline solids. 1994, 172, 715–720. [Google Scholar] [CrossRef]
- Laksmana, F.L.; Kok, P.J.A.H.; Vromans, H.; Van der Voort, K. Maarschalk, Predicting the diffusion coefficient of water vapor through glassy HPMC films at different environmental conditions using the free volume additivity approach. Eur. J. Pharm. Sci. 2009, 37, 545–554. [Google Scholar] [CrossRef]
- Robeson, L.M. The effect of antiplasticization on secondary loss transitions and permeability of polymers. Polym. Eng. Sci. 1969, 9, 277–281. [Google Scholar] [CrossRef]
- Maeda, Y.; Paul, D.R. Effect of antiplasticization on selectivity and productivity of gas separation membranes. J. Membr. Sci. 1987, 30, 1–9. [Google Scholar] [CrossRef]
- Guo, J.-H.; Robertson, R.E.; Amidon, G.L. Thermodynamic Aspects of the Disappearance of Antiplasticization in Slightly Plasticized Polymer Films at High Temperature. J. Pharm. Sci. 1992, 81, 1229–1230. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-H. A Theoretical and Experimental Study of Additive Effects of Physical Aging and Antiplasticization on the Water Permeability of Polymer Film Coatings. J. Pharm. Sci. 1994, 83, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Horn, N.R.; Paul, D.R. Carbon Dioxide Sorption and Plasticization of Thin Glassy Polymer Films Tracked by Optical Methods. Macromolecules 2012, 45, 2820–2834. [Google Scholar] [CrossRef]
- Xia, J.; Chung, T.-S.; Li, P.; Horn, N.R.; Paul, D.R. Aging and carbon dioxide plasticization of thin polyetherimide films. Polymer (Guildf) 2012, 53, 2099–2108. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Roth, C.B. (Ed.) Polymer Glasses; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Tian, Z.; Cao, B.; Li, P. Effects of sub-Tg cross-linking of triptycene-based polyimides on gas permeation, plasticization resistance and physical aging properties. J. Membr. Sci. 2018, 560, 87–96. [Google Scholar] [CrossRef]
- Kim, H.; Koros, W.J.; Paul, D.R. Physical aging of thin 6FDA-based polyimide membranes containing carboxyl acid groups. Part I. Transport properties. Polymer 2006, 47, 3094–3103. [Google Scholar] [CrossRef]
- Dorkenoo, K.D.; Pfromm, P.H. Accelerated Physical Aging of Thin Poly[1 -(trimethylsilyl)-1-propyne] Films. Macromolecules 2000, 33, 3747–3751. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Physical Aging of Thin Glassy Polymer Films Monitored by Optical Properties. Macromolecules 2006, 39, 1554–1559. [Google Scholar] [CrossRef]
- Vaughn, J.T.; Koros, W.J.; Johnson, J.R.; Karvan, O. Effect of thermal annealing on a novel polyamide–imide polymer membrane for aggressive acid gas separations. J. Membr. Sci. 2012, 401–402, 163–174. [Google Scholar] [CrossRef]
- Bernardo, P.; Bazzarelli, F.; Tasselli, F.; Clarizia, G.; Mason, C.R.; Maynard-Atem, L.; Budd, P.M.; Lanč, M.; Pilnáček, K.; Vopička, O.; et al. Effect of physical aging on the gas transport and sorption in PIM-1 membranes. Polymer 2017, 113, 283–294. [Google Scholar] [CrossRef]
- Burgess, S.K.; Lee, J.S.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Caffeine antiplasticization of amorphous poly(ethylene terephthalate): Effects on gas transport, thermal, and mechanical properties. Polymer 2015, 65, 34–44. [Google Scholar] [CrossRef]
- Liang, J.; Xia, Q.; Wang, S.; Li, J.; Huang, Q.; Ludescher, R.D. Influence of glycerol on the molecular mobility, oxygen permeability and microstructure of amorphous zein films. Food Hydrocoll. 2015, 44, 94–100. [Google Scholar] [CrossRef]
- Guo, J.H. Investigating the additive effects of physical aging and antiplasticization on the water permeability of polymer for controlled release film coatings. Control. Release Soc. 1993, 20, 324–325. [Google Scholar]
- Donempudi, S.; Yaseen, M. Controlled release PVC membranes: Influence of phthalate plasticizers on their tensile properties and performance. Polym. Eng. Sci. 1999, 39, 399–405. [Google Scholar] [CrossRef]
- Cava, D.; Sammon, C.; Lagaron, J.M. Sorption-induced release of antimicrobial isopropanol in EVOH copolymers as determined by ATR-FTIR spectroscopy. J. Appl. Polym. Sci. 2007, 103, 3431–3437. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R. Characterization of ethylene-vinyl alcohol copolymer containing lauril arginate (LAE) as material for active antimicrobial food packaging. Food Packag. Shelf Life 2014, 1, 10–18. [Google Scholar] [CrossRef]
- Rezus, Y.L.A.; Bakker, H.J. Effect of urea on the structural dynamics of water. Proc. Natl. Acad. Sci. 2006, 103, 18417–18420. [Google Scholar] [CrossRef] [Green Version]
- Van der Sman, R.G.M. Phase separation, antiplasticization and moisture sorption in ternary systems containing polysaccharides and polyols. Food Hydrocoll. 2019, 87, 360–370. [Google Scholar] [CrossRef]
- Lodagekar, A.; Chavan, R.B.; Chella, N.; Shastri, N.R. Role of Valsartan as an Antiplasticizer in Development of Therapeutically Viable Drug–Drug Coamorphous System. Cryst. Growth Des. 2018, 18, 1944–1950. [Google Scholar] [CrossRef]
- Chamarthy, S.P.; Pinal, R. Plasticizer concentration and the performance of a diffusion-controlled polymeric drug delivery system. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 25–30. [Google Scholar] [CrossRef]
- Cicerone, M.T.; Soles, C.L. Fast dynamics and stabilization of proteins: Binary glasses of trehalose and glycerol. Biophys. J. 2004, 86, 3836–3845. [Google Scholar] [CrossRef] [Green Version]
- Cicerone, M.T.; Tellington, A.; Trost, L.; Sokolov, A.P. Substantially improved stability of biological agents in dried form. Bioprocess Int. 2003, 1, 36–47. [Google Scholar]
- Chokshi, R.J.; Shah, N.H.; Sandhu, H.K.; Malick, A.W.; Zia, H. Stabilization of Low Glass Transition Temperature Indomethacin Formulations: Impact of Polymer-Type and its Concentration. J. Pharm. Sci. 2008, 97, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Labuza, T.P.; Labuza, T.J.; Labuza, K.M.; Labuza, P.S. Soft Condensed Matter: A Perspective on the Physics of Food States and Stability. In Water Properties in Food, Health, Pharmaceutical and Biological Systems: ISOPOW 10; Reid, D.S., Sajjaanantakul, T., Lillford, P.J., Charoenrein, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Chapter 7. [Google Scholar]
- Pittia, P.; Sacchetti, G.; Rocculi, P.; Venturi, L.; Rosa, M.C.M.D. Water State and Mobility Affect the Mechanical Properties of Coffee Beans. In Water Properties in Food, Health, Pharmaceutical and Biological Systems: ISOPOW 10; Reid, D.S., Sajjaanantakul, T., Lillford, P.J., Charoenrein, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Chapter 41. [Google Scholar]
- Farroni, A.; Matiacevich, S.B.; Guerrero, S.; Alzamora, S.; Buera, M.P. Thermal Transitions, Mechanical Properties, and Molecular Mobility in Cornflakes as Affected by Water Content. In Water Properties in Food, Health, Pharmaceutical and Biological Systems: ISOPOW 10; Reid, D.S., Sajjaanantakul, T., Lillford, P.J., Charoenrein, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Chapter 41. [Google Scholar]
- Wu, C.; Xu, W. Atomistic simulation study of absorbed water influence on structure and properties of crosslinked epoxy resin. Polymer (Guildf) 2007, 48, 5440–5448. [Google Scholar] [CrossRef]
- Ubbink, J. Carbohydrates in Amorphous States: Molecular Packing, Nanostructure, and Interaction with Water. In Water Properties in Food, Health, Pharmaceutical and Biological Systems: ISOPOW 10; Reid, D.S., Sajjaanantakul, T., Lillford, P.J., Charoenrein, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Chapter 26. [Google Scholar]
- Cuarmathy, S.P.; Pinal, R. Another unusual property of water: It increases the Tg of a glassy polymer. In Water Properties in Food, Health, Pharmaceutical and Biological Systems: ISOPOW 10; Reid, D.S., Sajjaanantakul, T., Lillford, P.J., Charoenrein, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Chapter 29. [Google Scholar]
- Ruiz, G.N.; Romanini, M.; Hauptmann, A.; Loerting, T.; Shalaev, E.; Tamarit, J.L.; Pardo, L.C.; Macovez, R. Genuine antiplasticizing effect of water on a glass-former drug. Sci. Rep. 2017, 7, 7470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, E.; Sandoval, A.J.; Cova, A.; Müller, A.J. Anti-plasticization of cassava starch by complexing fatty acids. Carbohydr. Polym. 2013, 98, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Iriartea, M.; Uriartea, C.; Iruina, J.J.; Etxeberriaa, A.; del Rio, J. Antiplasticization of a polyamide: A positron annihilation lifetime spectroscopy study. Polymer 2004, 45, 2949–2957. [Google Scholar] [CrossRef]
- Casalini, R.; Ngai, K.L.; Robertson, C.G.; Roland, C.M. α- and β-Relaxations in neat and antiplasticized polybutadiene. J. Polym. Sci. B Polym. Phys. 2000, 38, 1841–1847. [Google Scholar] [CrossRef]
- Andrews, G.P.; AbuDiak, O.A.; Jones, D.S. Physicochemical Characterization of Hot Melt Extruded Bicalutamide–Polyvinylpyrrolidone Solid Dispersions. J. Pharm. Sci. 2010, 99, 1322–1335. [Google Scholar] [CrossRef]
- Obrzut, J.; Anopchenko, A.; Douglas, J.F.; Rust, B.W. Relaxation and antiplasticization measurements in trehalose–glycerol mixtures—A model formulation for protein preservation. J. Non-Cryst. Solids 2010, 356, 777–781. [Google Scholar] [CrossRef]
- Curtis, J.E.; Dirama, T.E.; Carri, G.A.; Tobias, D.J. Inertial suppression of protein dynamics in a binary glycerol trehalose glass. J. Phys. Chem. B 2006, 110, 22953–22956. [Google Scholar] [CrossRef]
- Schäfer, H.; Sternin, E.; Stannarius, R.; Arndt, M.; Kremer, F. Novel Approach to the Analysis of Broadband Dielectric Spectra. Phys. Rev. Lett. 1996, 76, 2177–2180. [Google Scholar] [CrossRef]
- Ubbink, J. Structural and thermodynamic aspects of plasticization and antiplasticization in glassy encapsulation and biostabilization matrices. Adv. Drug Deliv. Rev. 2016, 100, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Anopchenko, A.; Psurek, T.; VanderHart, D.; Douglas, J.F.; Obrzut, J. Dielectric study of the antiplasticization of trehalose by glycerol. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2006, 74, 031501. [Google Scholar] [CrossRef] [PubMed]
- Riggleman, R.A.; de Pablo, J.J. Antiplasticization and local elastic constants in trehalose and glycerol mixtures. J. Chem. Phys. 2008, 128, 224504. [Google Scholar] [CrossRef] [PubMed]
- Roe, K.D.; Labuza, T.P. Glass transition and crystallization of amorphous trehalose-sucrose mixtures. Int. J. Food Prop. 2005, 8, 559–574. [Google Scholar] [CrossRef]
- Weng, L.; Elliott, G.D. Local Minimum in Fragility for Trehalose/Glycerol Mixtures: Implications for Biopharmaceutical Stabilization. J. Phys. Chem. B 2015, 119, 6820–6827. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Saedi, S.; Madaeni, S.S.; Shamsabadi, A.A. Fabrication of asymmetric polyethersulfone membranes for separation of carbon dioxide from methane using polyetherimide as polymeric additive. Chem. Eng. Res. Des. 2014, 92, 2431–2438. [Google Scholar] [CrossRef]
- Modesti, M.; Dall’Acqua, C.; Lorenzetti, A.; Florian, E. Mathematical model and experimental validation of water cluster influence upon vapour permeation through hydrophilic dense membrane. J. Membr. Sci. 2004, 229, 211–223. [Google Scholar] [CrossRef]
- Marais, S.; Metayer, M.; Nguyen, T.Q.; Labbe, M.; Saiter, J.M. Diffusion and permeation of water through unsaturated polyester resins—Influence of resin curing. Eur. Polym. J. 2000, 36, 453–462. [Google Scholar] [CrossRef]
- Ward, J.K.; Koros, W.J. Crosslinkable mixed matrix membranes with surface modified molecular sieves for natural gas purification: II. Performance characterization under contaminated feed conditions. J. Membr. Sci. 2011, 377, 82–88. [Google Scholar] [CrossRef]
- Kamaruddin, H.D. Some observations about the application of Fick’s first law for membrane separation of multicomponent mixtures. J. Membr. Sci. 1997, 135, 147–159. [Google Scholar] [CrossRef]
- Rasoldier, N.; Colin, X.; Verdu, J.; Bocquet, M.; Olivier, L.; Chocinski-Arnault, L.; Lafarie-Frenot, M.C. Model systems for thermo-oxidised epoxy composite matrices. Compos. Part A 2008, 39, 1522–1529. [Google Scholar] [CrossRef]
- Vassileva, E.; Friedrich, K. Epoxy/Alumina Nanoparticle Composites. I. Dynamic Mechanical Behavior. J. App. Polym. Sci. 2003, 89, 3774–3785. [Google Scholar] [CrossRef]
- Mascia, L.; Coroli, A.; Mele, E. Probing the Thermal transitions of lactobionic acid and effects of sample history by DSC analysis. J. Pharm. Sci. 2019, 108, 3781–3784. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S.; Herald, T.J. Effects of plasticizers on the structure and properties of starch–clay nanocomposite films. Carbohydr. Polym. 2008, 74, 552–558. [Google Scholar] [CrossRef]
- Michaelis, M.; Brummer, R.; Leopold, C.S. Plasticization and antiplasticizatio.n of an acrylic pressure sensitive adhesive by ibuprofen and their effect on the adhesion properties. Eur. J. Pharm. Biopharm. 2014, 86, 234–243. [Google Scholar] [CrossRef]
- Madrigal, L.; Sandoval, A.J.; Müller, A.J. Effects of corn oil on glass transition temperatures of cassava starch. Carbohydr. Polym. 2011, 85, 875–884. [Google Scholar] [CrossRef]
- Dubault, A.; Bokobza, L.; Gandin, E.; Halary, J.L. Effects of molecular interactions on the viscoelastic and plastic behaviour of plasticized poly(vinyl chloride). Polym. Int. 2003, 52, 1108–1118. [Google Scholar] [CrossRef]
- Daly, J.; Britten, A.; Garton, A.; McLean, P.D. An additive for increasing the strength and modulus of amine-cured epoxy resins. J. Appl. Polym. Sci. 1984, 29, 1403–1414. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A.; Coma, V. Effects of Hydrophilic Plasticizers on Mechanical, Thermal, and Surface Properties of Chitosan Films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Das, S.; Biswas, R. Density relaxation and particle motion characteristics in a non-ionic deep eutectic solvent (acetamide + urea): Time-resolved fluorescence measurements and all-atom molecular dynamics simulations. J. Chem. Phys. 2015, 142, 034505. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, J. The Effects of Plasticizers Containing Amide Groups on the Properties of Thermoplastic Starch. Starch-Stärke 2004, 56, 545–551. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Souza, H.K.S.; Latona, N.; Liu, C.-K.; Gonçalves, M.P.; Liu, L. Choline chloride based ionic liquid analogues as tool for the fabrication of agar films with improved mechanical properties. Carbohydr. Polym. 2014, 111, 206–214. [Google Scholar] [CrossRef]
- Vashchuk, A.; Fainleib, A.M.; Starostenko, O.; Grande, D. Application of ionic liquids in thermosetting polymers: Epoxy and cyanate ester resins. Express Polym. Lett. 2018, 12, 898–917. [Google Scholar] [CrossRef]
- Lionetto, F.; Mascia, L.; Frigione, M. Evolution of Transient-states and Properties of anEpoxy-Silica Hybrid cured at Ambient Temperature. Eur. Polym. J. 2013, 49, 1298–1313. [Google Scholar] [CrossRef]
- Murphy, L.; Sun, B.; Hong, W.; Aziz, H.; Li, Y. Study of Vertical and Lateral Charge Transport Properties of DPP-Based Polymer/PC61BM Films Using Space Charge Limited Current (SCLC) and Field Effect Transistor Methods and their Effects on Photovoltaic Characteristics. Aust. J. Chem. 2015, 68, 1741. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Ford, E. Antiplasticizing Behaviors of Glucarate and Lignin Bio-Based Derivatives on the Properties of Gel-Spun Poly(Vinyl Alcohol) Fibers. Macromol. Mater. Eng. 2018, 303, 1700523. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Tongsin, P.; Intasanta, N.; Yoovidhya, T. Effect of glycerol on solution properties governing morphology, glass transition temperature, and tensile properties of electrospun zein fibers. J. Appl. Polym. Sci. 2010, 18, 910–919. [Google Scholar]
- Mascia, L.; Zhang, W.; Gatto, F.; Scarpellini, A.; Pompa, P.P.; Mele, E. In situ generation of ZnO nanoparticles within a polyethyleneimine matrix for antibacterial zein fibres. ACS Appl. Polym. Mater. 2019, 1, 1707–1716. [Google Scholar] [CrossRef]











| Material | δ Values (MJ∙m−3)1/2 | ||
|---|---|---|---|
| Dispersive | Dipolar | H-Bonding | |
| PVC (Poly vinyl chloride) | 18.4 | 11.1 | 1.9 |
| TCP (Tri-cresyl phosphate) | 19.0 | 12.3 | 4.5 |
| DOP (Bis-2-ethylhexyl phthalate) | 16.6 | 8.0 | 3.1 |
| PA 11 (Polyundecanoiamide) | 18.1 | 5.1 | 11.2 |
| BBSA (N-Butylbenzene sulphonamide) | 18.9 | 7.9 | 8.8 |
| GLY (Glycerol) | 17.3 | 12.1 | 29.3 |
| SBO (Soybean oil) | 17.1 | 2.5 | 2.8 |
| Water Content (w%) | ψsp(ω) (cm3/g) | ψ*sp (cm3/g) | Δ(FV) (%) | Physical State of System |
|---|---|---|---|---|
| 0.00 | 0.6675 | - | 0.00 | Pure polymer |
| 1.35 | 0.6669 | 0.6720 | −0.76 | Bound water regime |
| 2.75 | 0.6620 | 0.6766 | −2.16 | Bound water regime |
| 5.98 | 0.6642 | 0.6874 | −3.38 | Mixed free/bound water regime |
| 10.50 | 0.6688 | 0.7024 | −4.96 | Plasticization threshold |
| 15.20 | 0.6798 | 0.7180 | −5.32 | Plasticization regime |
| 19.50 | 0.6926 | 0.7323 | −5.42 | Plasticization regime |
| 26.10 | 0.7118 | 0.7539 | −5.59 | Plasticization regime |
| 33.00 | 0.7368 | 0.7783 | −5.50 | Plasticization regime |
| Plasticizer Content | PPO/TCP (@ 25 °C) | PPO/DOP (@ 25 °C) | Starch/Water (@ 30 °C) | |||
|---|---|---|---|---|---|---|
| (wt.%) | Δ(Ve) | Δ(FV) (%) | Δ(Ve) | Δ(FV) (%) | Δ(Ve) | Δ(FV) (%) |
| 6 | - | - | - | - | −0.023 | −3.4 |
| 10 | −0.016 | −1.75 | −0.021 | −2.22 | −0.042 | −4.9 |
| 20 | −0.028 | −3.09 | −0.033 | −3.47 | −0.040 | −5.4 |
| 30 | −0.037 | −4.12 | −0.048 | −5.01 | −0.034 | −5.4 |
| Topic | Main features and Comments | References |
|---|---|---|
| Structure and physical state | Molecular interactions by FTIR: Upward shift in frequency wave number. | [33,40,41,42] |
| Free volumes: Deviation from additivity rule with a maximum extending into plasticization region. Transition antiplasticization to plasticization through the formation of plasticizer clusters. | [33,55,57,117,144,145] | |
| Relaxation spectra: Reduction in glass transition temperature with a depression of β relaxations peak through an increase in relaxation time, resulting in a reduction of the glass formation fragility. | [10,36,73,74,75,76,77,78,79,84,141] | |
| Mechanical properties | Modulus and Yield strength: Both increase over a temperature range between α and β peak transitions. Embrittlement: Results from a reduction in crazing strain and fracture toughness between α and β peak transitions. | [9,10,12,15,77,86,87,123] |
| Diffusion related properties | Gas permeation barrier: Monotonic reduction in gas permeation rate with increasing plasticizer concentration to a minimum, followed by a rapid increase within the plasticization regime. Sorption and Release: Reduction of water absorption of carbohydrates in presence of plasticizers exerting strong interactions with the polymer chains. | [17,32,55,57,99,110,111,112,116,117,144] |
| Related aspects | Physical ageing: Specific volume variation with temperature is reduced, accompanied by a small increase in Tg. Plasticizers accelerate physical ageing. | [9,11,59,61,66,72,98,99,102,103,104] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascia, L.; Kouparitsas, Y.; Nocita, D.; Bao, X. Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties. Polymers 2020, 12, 769. https://doi.org/10.3390/polym12040769
Mascia L, Kouparitsas Y, Nocita D, Bao X. Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties. Polymers. 2020; 12(4):769. https://doi.org/10.3390/polym12040769
Chicago/Turabian StyleMascia, Leno, Yannis Kouparitsas, Davide Nocita, and Xujin Bao. 2020. "Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties" Polymers 12, no. 4: 769. https://doi.org/10.3390/polym12040769