1. Introduction
Polymeric nanocomposites, i.e., polymers with nanofillers incorporated into the matrix, have been intensively studied during the last decades. The extremely large specific surface area of all nanofillers can be exploited to improve many material properties. Traditional composites are typically designed for improving the mechanical properties of the material, but nanocomposites are more versatile; for instance, the thermal and electrical properties are also influenced by the addition of nanofillers such as graphene, ZnO, or Al
2O
3 [
1,
2].
A good dispersion of the nanoparticles is generally required to maximize the effective particle surface area and to achieve a homogenous material with few detrimental material flaws. Ultra-sonication of the nanoparticles can be performed prior to mixing to break-up particle agglomerates and facilitate good particle dispersion. Efficient mechanical mixing during the nanocomposite manufacturing is also desired. The dispersion is further influenced by the volume filler fraction of particles and by the predisposition of the particles to self-assemble and form agglomerates within the matrix; nanoparticles are typically prone to aggregate due to their large surface area. Moreover, if hydrophilic inorganic nanoparticles, such as SiO
2, Al
2O
3, or ZnO, are introduced directly into a hydrophobic polymer matrix, the particle miscibility and the adhesion between the particles and the matrix can easily become unsatisfying. Even though a proper surface modification of the nanofillers can significantly improve the dispersion [
2,
3], it is still a major challenge for scientists and material producers to obtain completely controlled dispersions.
Field grading materials (FGMs) are defined as materials with a resistivity depending on the applied electrical field (
E-field). In particular, non-linear resistive FGMs have an electrical response that rapidly switches from insulating to conducting when the
E-field is increased above a threshold level. Such materials can be used to minimize the risk of electrical breakdown due to local
E-field enhancements in crucial electrical accessories such as high-voltage direct-current (HVDC) cable joints and cable terminations. Currently available nonlinear FGMs are typically rubber-based, percolated composites filled with approximately 30–40 vol.% particles [
4,
5,
6]. The high particle filler fractions may result in unnecessarily heavy and brittle composites.
Graphene, graphene oxide (GO), and (partially) reduced graphene oxide (rGO) have interesting electrical properties [
2,
7,
8,
9,
10,
11] and have therefore attracted attention as promising fillers for the next generation of non-linear resistive FGMs [
2,
7,
8,
9,
10,
11]. For instance, the addition of 2.5 vol.% partially reduced GO into a silicone rubber matrix and resulted in a material with non-linear resistivity [
12]. In this study, the abbreviation rGO is used, for simplicity, both for partially and completely reduced GO. The reduction of GO, either by thermal treatment [
13] or chemical procedures [
14], reduces the oxygen fraction of the nano-flakes. A higher reduction degree of the rGO therefore corresponds to a higher conductivity of the rGO flakes. The electrical properties of a completely reduced rGO are thus close to the properties of pure graphene. When GO is thermally reduced in air at 120 °C, as in this study, the removed oxygen atoms mainly come from C–O and C–OH bonds, while the oxygen from C=O and O=C-OH groups remain nearly unaffected [
13].
In a recent study [
2], the addition of 2 vol.% poly(
n-butyl methacrylate) surface-modified rGO (rGO-PBMA) in a poly(ethylene-
co-butyl acrylate) (EBA) matrix enabled a pronounced non-linear resistivity. Three different polymer graft lengths (10, 50, and 70 kg mol
−1) were used to improve the compatibility between the rGO and the EBA matrix and to avoid aggregation of the filler. The rGO-PBMA with the shortest polymer grafts showed a rapid and sharp non-linear resistivity above the
E-field threshold. The resistivity results were explained by three observations: (1) since the composites were isotropic with filler fractions locally higher than the predicted electrical percolation threshold, longer graft lengths resulted in longer interparticle distances, (2) the average interparticle distance was close to the maximum charge-carrier hopping distance, and (3) an increased
E-field increased the hopping distance. Therefore, a field-grading non-linear resistivity was expected with increasing electrical field, which was also observed.
This work is an extension of the previous rGO-PBMA/EBA study [
2]. A slightly updated nanocomposite preparation technique is here utilized, enabling a higher degree of control and predictability of the composite resistivity. EBA-based nanocomposites with 2, 3, and 4 vol.% of rGO and rGO-PBMAs, respectively, were prepared by solvent casting in
p-xylene. Three comparatively short graft-lengths of 3.0, 4.5, and 9.2 kg/mol were chosen to further decrease the inter-flake distance compared to our previous study. The morphology and resistivity of the composites were analyzed both with SEM, resistivity measurements, and Monte-Carlo computer simulations in order to reveal how the non-linear composite resistivity is influenced by the dispersion and orientation of the rGO flakes. The aim of this work was to develop light and stable FGMs, with tuneable electrical nonlinearities and improved mechanical properties, for HVDC applications.
2. Experimental Section
2.1. Materials
Poly(ethylene-co-butyl acrylate) pellets (EBA, Mw = 205 kDa, 27% BA) were supplied by Borealis AB (Stenungssund, Sweden). Freeze-dried GO was purchased from Abalonyx (Oslo, Norway) and homogenized in ethanol:water mixtures (1:1 v/v), purified and partially reduced by thermal reduction in air at 120 °C for 20 h prior to use or surface modification. Chemicals were used as received from Sigma-Aldrich unless stated otherwise; (3-aminopropyl)triethoxysilane (APTES, 98%), n-butyl methacrylate (BMA, 97%), 2-bromoisobutyryl bromide (α-BiB, 98%), ethyl α-bromoisobutyrate (EBiB) (98%), copper(I) bromide (Cu(I)Br, 98%), copper(II) bromide (Cu(II)Br2, 99%), 4-(dimethylamino) pyridine (DMAP, 99%), triethylamine (TEA, 99%), and 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA, 97%). Butyl methacrylate (BMA, 96%) was destabilized prior to use by passing it through a column of Al2O3 (neutral). Deionized water, ethanol (EtOH, 96%), tetrahydrofuran (THF, analytical grade), dichloromethane (DCM, analytical grade), methanol (HPLC-grade), diethyl ether (HPLC-grade), p-xylene, and toluene (HPLC-grade) were used without further purification.
2.2. Procedures to Modify GO: Thermal Reduction, Silanization, Attachment of Initiator, and Surface-Initiated Atom-Transfer Radical Polymerization (SI-ATRP) of PBMA (rGO, rGO-Silanized, rGO-Initiated and rGO-PBMAs)
The procedures used to obtain rGO-Silanized, rGO-Initiated, and rGO-PBMAs are published elsewhere [
2] and only summarized below.
GO (500 mg) was added to a round bottom flask, with EtOH (150 mL), equipped with a magnet stir bar. Deionized water (150 mL) was added and the mixture was ultrasonicated for 5 min and thereafter stirred overnight. The suspension was purified with EtOH:water (1:1
v/
v) twice, by centrifugation (20 min at 21,000 g) and ultrasonication (5 min) cycles. Finally, the GO was freeze dried and thermally reduced in an oven at 120 °C for 20 h, obtaining partially reduced graphene oxide (rGO). This partial reduction process decreases the oxygen content from approximately 31.5% (GO) to 19.7% (rGO) [
13].
In order to obtain rGO-Silanized, EtOH (225 mL) was added to rGO (500 mg). Deionized water (100 mL) was added to the suspension and the mixture was ultrasonicated (5 min in bath and 30 s, 30% amplitude with 8/2 sec on/off pulses). Then, (3-Aminopropyl)triethoxysilane (APTES, 15 mL, 200 mmol) was added dropwise to the suspension at room temperature (RT), and subsequently the reaction continued under reflux at 80 °C overnight. The final rGO-Silanized suspension was purified by three centrifugation and ultrasonication cycles in EtOH:water (2:1) and finally twice in THF.
To create rGO-Initiated (~500 g) suspended in THF (60 mL), α-bromoisobutyryl bromide (0.97 g, 4.2 mmol α-BiB), trimethylamine (TEA, 0.60 g, 4.9 mmol), and 4-(dimethylamino)pyridine (DMAP, 5 grains) were added and left on a shaking table overnight. A small amount of EtOH was added to quench the reaction before purification of rGO-Initiated. The final material was purified by successive centrifugation–homogenization (ultrasonication) cycles in THF:EtOH, THF, DCM, and finally twice in toluene. The final rGO-Initiated was suspended in toluene (20 mL).
Surface-initiated atom-transfer radical polymerization (SI-ATRP) was used to graft PBMA polymer chains from the rGO-Initiated surfaces. rGO-Initiated (70 mg), toluene (15 mL), destabilized n-butyl methacrylate (BMA, 62.8 mmol, 8.93 g), 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA, 24.5 µL, 90 µmol), and ethyl α-bromoisobutyrate (EBiB, 11.8 µL, 81 µmol) were added to a round bottom flask equipped with a stirrer and degassed by 2 vacuum/argon cycles (5 + 5 min). Subsequently, copper(I) bromide (CuBr, 10 mg, 72 µmol) and copper(II) bromide (CuBr2, 4.0 mg, 18 µmol) were added and two extra degassing cycles performed. The reaction was placed in an oil bath preheated to 60 °C and left to react for 2, 4, or 6 h, to yield rGO-PBMA-3K, rGO-PBMA-4.5K and rGO-PBMA-9K. The rGO-PBMAs were purified by successive centrifugation and ultra-sonication cycles in THF (three times), using the first supernatant to recover the free PBMA formed from the sacrificial initiator during the polymerization and characterized by DMF-SEC.
FT-IR and TGA were used to characterize the rGO, rGO, rGO-Silanized, rGO-Initiated, and rGO-PBMAs.
2.3. Nanocomposite Formation from rGO-PBMAs and the EBA Matrix
The rGO and rGO-PBMAs were used in 2, 3, and 4 vol.% (4, 6, and 8 wt %) in an EBA matrix. First, the required amounts of filler were weighed in vials and dispersed in p-xylene (5 mL) using the ultrasonication bath (10 min). EBA (200 mg) was weighed in a vial equipped with a magnetic stirrer, and subsequently added to the rGO dispersion. The vial was then placed in an oil bath preheated to 115 °C, and the reaction mixture was stirred for 1 h. The mixture was poured in a flask and left to dry at 90 °C overnight in an oven, followed by another two days drying at 60 °C. The resulting nanocomposites were hot-pressed into discs (3.4 cm diameter, 148 ± 7 µm thick, 150 °C, 145 kN, 20 min). Due to the highly polished surfaces of the press plates, the largest observed thickness variation within an individual disc was only 2.9%. The samples were finally characterized with SEM and resistivity measurements.
2.4. Instrumentation and Characterization Methods
Size exclusion chromatography (SEC) was performed using dimethylformamide (DMF) (0.2 mL·min−1) as the mobile phase at 50 °C, using a TOSOH EcoSEC HLC-8320GPC system equipped with an EcoSEC RI detector and three columns (PSS PFG 5 μm; Microguard, 100 Å, and 300 Å) (MW resolving range: 300–100,000 Da) from PSS GmbH. A conventional calibration method was created using narrow linear poly(methyl methacrylate) (PMMA) standards. Corrections for flow rate fluctuations were made using toluene as an internal standard. PSS WinGPC Unity software version 7.2 was used to process data.
Fourier transformation infrared spectroscopy, FT-IR, was performed with a Perkin-Elmer Spectrum 2000 FT-IR equipped with a MKII Golden Gate, single reflection ATR System (from Specac Ltd., London, U.K.). The ATR-crystal was a MKII heated Diamond 45° ATR Top Plate.
Thermogravimetric analysis (TGA) was performed with a TA Instruments Hi-Res TGA 2950 analyzer (TA Instruments, Newcastle, Ireland) under nitrogen flow. The heating rate was 10 °C/min and the experiments were performed from 40 to 700 °C. Two measurements were done for each material composition and the average was used in the plots.
Differential scanning calorimetry (DSC) was performed with a DSC 1 from Mettler-Toledo. Samples of 5–10 mg were measured through a cycle of heating–cooling–heating, with a starting temperature of −30 °C and up to 160 °C at a heating/cooling rate of 10 °C/min.
A Hitachi S-4800 field emission scanning electron microscope was used to characterize the fractured film surfaces.
Tensile tests of the composites were performed using a universal materials testing machine Instron 5944 (Instron., Norwood, MA, USA) equipped with a 500 N load cell. The samples were conditioned at 23 °C under controlled relative humidity of 50 RH% for at least one week prior to the tensile test. The samples were cut in strips (25 × 5 mm) and tested at a 50%/min strain rate. All the mechanical data reported were obtained from at least three specimens for each sample and the means and standard deviations were reported.
Resistivity measurements perpendicular to the film direction of the approximately 150 µm thick pressed plates were conducted in an oven (Binder FED 115) for maintaining a controlled temperature of 25 °C and additional shielding. A high voltage electrode of stainless steel and a guarded measurement electrode system of brass with a 20 mm diameter of the inner electrode were used. The electric field was increased with polarizing/depolarizing cycles of 12 min from 0.2 to 8 kV mm−1. The high voltage supply was a HCP-35-12500, FuG Elektronik (Schechen, Germany) and a 6517B electrometer, Keithley (Cleveland, Ohio, USA) was used to measure the current through the sample. The thickness of the individual specimen was used when converting the measured current to resistivity. Only one measurement was reported for each material composition, but additional replicate measurements were also performed, indicating good reproducibility.
4. Conclusions
Thermally reduced graphene oxide (rGO) sheets were successfully surface modified with PBMA using SI-ATRP. PBMA chains with molecular weights 3, 4.5, and 9 kg/mol were thus grafted from silanized and initiator immobilized rGO surfaces, to obtain PBMA-grafted reduced graphene oxide (rGO-PBMA). Short polymer grafts significantly increased the thermal stability of the nano-fillers and confirmed that the modification was successful. EBA nanocomposites with rGO and rGO-PBMAs were prepared by solvent casting and subsequently hot-pressed to create films. The thermal stability of the nanocomposites increased significantly by increasing the rGO filler fraction or by adding PBMA grafts, reaching a 70 °C increase at 4 vol.% rGO-PBMA. A probable explanation is that the rGO flakes impede diffusion of volatile combustion gases from the composite and thus stabilize the material.
SEM analysis of nanocomposite cross-sections revealed nanoflake dispersions with acceptable degrees of agglomeration, although a distinct directionality perpendicular to the pressing direction was observed in all samples, except for the rGO-PBMA-9K nanocomposites. Longer graft lengths result in extended interparticle distances which, in turn, facilitate the manufacturing of composites with isotropic particle orientations where the directionality is also limited after hot-pressing. The Young’s modulus of the nanocomposites increased upon addition of rGO, being more significant for the rGO-PBMA-4.5-4 vol.%, and the strain at maximum tensile stress of the rGO-PBMAs improved significantly at low loadings, indicating that the grafting of PBMA from the rGO increases the compatibility between the filler and the EBA matrix.
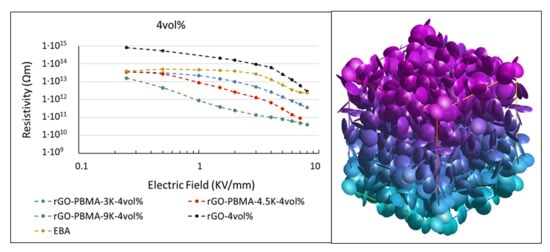
The SEM micrographs were used to measure the aspect ratios and orientations of the rGO sheets. These aspect ratios and orientations were inserted into a Monte-Carlo simulation model to predict the electrical percolation threshold as a function rGO orientation. A percolation threshold around 1–2 vol.% was predicted for nearly isotropic composites such as rGO-PBMA-9K and a corresponding threshold around 3–7 vol.% was predicted for more anisotropic composites, such as the composites with unmodified rGO. Below the threshold, the composite resistivities were expected to be similar to or higher than the EBA resistivity. Above the threshold the composite resistivities were expected to be lower and dependent on the length of the grafts separating the flakes. Field-dependent DC resistivity measurements were applied on the nanocomposites. At low filler fractions (2 wt %) the nanocomposites’ resistivities were slightly higher than that of the EBA reference, probably due to the trapping of electrons, ions, and polar molecules at the (well-separated) rGO surfaces. At higher filler fractions (3–4 vol.%), the PBMA-rGO/EBA resistivities were lower than that of the reference and decreased with increasing PBMA-graft length. Modeling suggests that an increasing PBMA length increases the interflake distances, which increases the resistivity. Simultaneously, the isotropy of the grafted rGO sheets increases, which subsequently decreases the resistivity. For sufficiently orientated rGO sheets with relatively short chains, the latter effect seems to dominate. This conclusion was supported by SEM images showing the highest isotropy in the nanocomposites with the longest PBMA-grafts.
To summarize, this study concludes that materials with E-field dependent resistivities can be obtained by dispersing small fractions (2–4 wt %) of PBMA-grafted rGO in EBA, which is in agreement with our previous results; the resistivity profile can be tailored towards desired applications by varying the rGO fraction and the PBMA graft length. Since these composites are lighter and have better mechanical properties than today’s field grading materials, they are promising candidates as field grading material in electrical HVDC applications.