Effects of Hydrothermal Pretreatment on the Structural Characteristics of Organosolv Lignin from Triarrhena lutarioriparia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
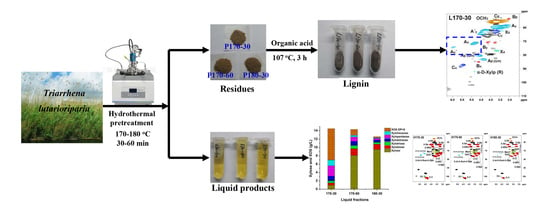
2.2. Fractionation Process
2.3. Preparation of DEL Samples from Raw and the Hydrothermally-Treated Substrates
2.4. Characterizations of the Isolated Products
3. Results and Discussion
3.1. Chemical Composition of the Substrates and Lignin
3.2. Characterizations of Liquid Products after Hydrothermal Pretreatment
3.3. The Effect of Hydrothermal Pretreatment on the Lignin Structure
3.4. The Effect of Hydrothermal Pretreatment on the Structural Features of Organosolv Lignin
3.5. Mass Balance of the Hydrothermal and Organic Acid Delignification Process
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cayetano, R.D.A.; Kim, T.H. Two-Stage Processing of Miscanthus Giganteus Using Anhydrous Ammonia and Hot Water for Effective Xylan Recovery and Improved Enzymatic Saccharification. Bioresour. Technol. 2018, 255, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Liu, Y.S.; Zeng, Y.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How Does Plant Cell Wall Nanoscale Architecture Correlate with Enzymatic Digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass Recalcitrance. Part II: Fundamentals of Different Pre-Treatments to Increase the Enzymatic Digestibility of Lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The Key to Unlocking Low-Cost Cellulosic Ethanol. Biofuels Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic Solvent Pretreatment of Lignocellulosic Biomass for Biofuels and Biochemicals: A Review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Inkrod, C.; Raita, M.; Champreda, V.; Laosiripojana, N. Characteristics of Lignin Extracted from Different Lignocellulosic Materials via Organosolv Fractionation. BioEnergy Res. 2018, 11, 277–290. [Google Scholar] [CrossRef]
- Zeng, X.; Jin, F.; Cao, J.; Yin, G.; Zhang, Y.; Zhao, J. Production of Formic Acid and Acetic Acid by Hydrothermal Oxidation of Alkali Lignin. AIP Conf. Proc. 2010, 1251, 384–387. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Hu, R.; Shupe, A.; Lin, L.; Liang, B. A Sustainable Woody Biomass Biorefinery. Biotechnol. Adv. 2012, 30, 785–810. [Google Scholar] [CrossRef] [PubMed]
- Abdelkafi, F.; Ammar, H.; Rousseau, B.; Tessier, M.; El Gharbi, R.; Fradet, A. Structural Analysis of Alfa Grass (Stipa tenacissima L.) Lignin Obtained by Acetic Acid/Formic Acid Delignification. Biomacromolecules 2011, 12, 3895–3902. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Microwave-Assisted Organic Acid Extraction of Lignin from Bamboo: Structure and Antioxidant Activity Investigation. Food Chem. 2012, 134, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Woody Biomass: Niche Position as a Source of Sustainable Renewable Chemicals and Energy and Kinetics of Hot-Water Extraction/Hydrolysis. Biotechnol. Adv. 2010, 28, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tao, L.; Wyman, C.E. Strengths, Challenges, and Opportunities for Hydrothermal Pretreatment in Lignocellulosic Biorefineries. Biofuels Bioprod. Biorefin. 2018, 12, 125–138. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.N.; Yuan, T.Q.; Xu, F.; Sun, R.C. Fractionation of Bamboo Culms by Autohydrolysis, Organosolv Delignification and Extended Delignification: Understanding the Fundamental Chemistry of the Lignin during the Integrated Process. Bioresour. Technol. 2013, 150, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Pihlajaniemi, V.; Sipponen, M.H.; Pastinen, O.; Lehtomäki, I.; Laakso, S. Yield Optimization and Rational Function Modelling of Enzymatic Hydrolysis of Wheat Straw Pretreated by Naoh-Delignification, Autohydrolysis and Their Combination. Green Chem. 2015, 17, 1683–1691. [Google Scholar] [CrossRef]
- Sun, S.; Wen, J.; Sun, S.; Sun, R.C. Systematic Evaluation of the Degraded Products Evolved from the Hydrothermal Pretreatment of Sweet Sorghum Stems. Biotechnol. Biofuels 2015, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Wen, J.L.; Wang, B.; Wang, H.M.; Liu, C.F.; Sun, R.C. Assessment of Integrated Process Based on Autohydrolysis and Robust Delignification Process for Enzymatic Saccharification of Bamboo. Bioresour. Technol. 2017, 244, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Li, J.; Zhang, P.; Nabi, M.; Jin, S.; Li, F.; Wang, S.; Ye, J. Reinforced Acid-Pretreatment of Triarrhena Lutarioriparia to Accelerate Its Enzymatic Hydrolysis. Int. J. Hydrog. Energy 2017, 42, 18301–18308. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wang, B.; Wu, Y.Y.; Wen, J.L.; Liu, C.F.; Yuan, T.Q.; Sun, R.C. Structural Variations of Lignin Macromolecule from Different Growth Years of Triploid of Populus Tomentosa Carr. Int. J. Biol. Macromol. 2017, 101, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Li, H.-Y.; Wang, B.; Wen, J.-L.; Cao, X.-F.; Sun, S.-N.; Sun, R.-C. Availability of Four Energy Crops Assessing by the Enzymatic Hydrolysis and Structural Features of Lignin before and after Hydrothermal Treatment. Energy Convers. Manag. 2018, 155, 58–67. [Google Scholar] [CrossRef]
- Gullón, B.; Yáñez, R.; Alonso, J.L.; Parajó, J.C. Production of Oligosaccharides and Sugars from Rye Straw: A Kinetic Approach. Bioresour. Technol. 2010, 101, 6676–6684. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.; DomíNguez, H.; Parajó, J.C. Generation of Xylose Solutions from Eucalyptus Globulus Wood by Autohydrolysis-Posthydrolysis Processes: Posthydrolysis Kinetics. Bioresour. Technol. 2001, 79, 155–164. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J. A Gel-State 2d-Nmr Method for Plant Cell Wall Profiling and Analysis: A Model Study with the Amorphous Cellulose and Xylan from Ball-Milled Cotton Linters. RSC Adv. 2014, 4, 7549–7560. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Sun, R.-C. Structural Elucidation of Whole Lignin from Eucalyptus Based on Preswelling and Enzymatic Hydrolysis. Green Chem. 2015, 17, 1589–1596. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4,4,5,5-Tetramethyl-1,3,2-Dioxaphospholane, a Reagent for the Accurate Determination of the Uncondensed and Condensed Phenolic Moieties in Lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Wang, Z.W.; Zhu, M.Q.; Li, M.F.; Wang, J.Q.; Wei, Q.; Sun, R.C. Comprehensive Evaluation of the Liquid Fraction during the Hydrothermal Treatment of Rapeseed Straw. Biotechnol. Biofuels 2016, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Effect of Inhibitors Released during Steam-Explosion Treatment of Poplar Wood on Subsequent Enzymatic Hydrolysis and Ssf. Biotechnol. Prog. 2004, 20, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Vancov, T.; Mcintosh, S. Mild Acid Pretreatment and Enzyme Saccharification of Sorghum Bicolor Straw. Appl. Energy 2012, 92, 421–428. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, S.D.; Kim, H.; Lu, F.; Ralph, J. Whole Plant Cell Wall Characterization Using Solution-State 2d Nmr. Nat. Protoc. 2012, 7, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Jaaskelainen, A.S.; Sun, Y.; Argyropoulos, D.S.; Tamminen, T.; Hortling, B. The Effect of Isolation Method on the Chemical Structure of Residual Lignin. Wood Sci. Technol. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Quantitative Structural Characterization of the Lignins from the Stem and Pith of Bamboo (Phyllostachys pubescens). Holzforschung 2013, 67, 613–627. [Google Scholar] [CrossRef]
- Leschinsky, M.; Zuckerstätter, G.; Weber, H.K.; Patt, R.; Sixta, H. Effect of Autohydrolysis of Eucalyptus Globulus Wood on Lignin Structure. Part 1: Comparison of Different Lignin Fractions Formed during Water Prehydrolysis. Holzforschung 2008, 62, 645–652. [Google Scholar] [CrossRef]
- Leschinsky, M.; Zuckerstätter, G.; Weber, H.K.; Patt, R.; Sixta, H. Effect of Autohydrolysis of Eucalyptus Globulus Wood on Lignin Structure. Part 2: Influence of Autohydrolysis Intensity. Holzforschung 2008, 62, 653–658. [Google Scholar] [CrossRef]
- Okuda, N.; Hori, K.; Sato, M. Chemical Changes of Kenaf Core Binderless Boards during Hot Pressing (I): Influence of the Pressing Temperature Condition. J. Wood Sci. 2006, 52, 244–248. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Lin, Z.; Peng, W.-X.; Yuan, T.-Q.; Xu, F.; Li, N.-C.; Tao, Q.-S.; Xiang, H.; Sun, R.-C. Unraveling the Structural Characteristics of Lignin in Hydrothermal Pretreated Fibers and Manufactured Binderless Boards from Eucalyptus Grandis. Sustain. Chem. Process. 2014, 2, 9–20. [Google Scholar] [CrossRef]
- Korntner, P.; Sumerskii, I.; Bacher, M.; Rosenau, T.; Potthast, A. Characterization of Technical Lignins by Nmr Spectroscopy: Optimization of Functional Group Analysis by 31p Nmr Spectroscopy. Holzforschung 2015, 69, 807–814. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, A.N.T.; Ralph, J.; Gutiérrez, A. Structural Characterization of Wheat Straw Lignin as Revealed by Analytical Pyrolysis, 2d-Nmr, and Reductive Cleavage Methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Ogale, A.A. Carbon Fibers from Dry-Spinning of Acetylated Softwood Kraft Lignin. Carbon 2014, 69, 626–629. [Google Scholar] [CrossRef]
- Pizzi, A.; Zhou, X.; Navarrete, P.; Segovia, C.; Mansouri, H.R.; Placentia Pena, M.I.; Pichelin, F. Enhancing Water Resistance of Welded Dowel Wood Joints by Acetylated Lignin. J. Adhes. Sci. Technol. 2013, 27, 252–262. [Google Scholar] [CrossRef]
- Lohr, T.L.; Li, Z.; Marks, T.J. Selective Ether/Ester C–O Cleavage of an Acetylated Lignin Model via Tandem Catalysis. ACS Catal. 2015, 5, 7004–7007. [Google Scholar] [CrossRef]
- Löfstedt, J.; Dahlstrand, C.; Orebom, A.; Meuzelaar, G.; Sawadjoon, S.; Galkin, M.V.; Agback, P.; Wimby, M.; Corresa, E.; Mathieu, Y. Green Diesel from Kraft Lignin in Three Steps. Chemsuschem 2016, 9, 1392–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, Y.Q.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the Molecular Structure Basis for Biomass Recalcitrance during Dilute Acid and Hydrothermal Pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [PubMed]




| Name | Sugar/% | Ara a | Gal a | Glu a | Xyl a | Man a | Glua a |
|---|---|---|---|---|---|---|---|
| DEL | 18.42 | 1.90 | 0.40 | 4.05 | 10.14 | 1.32 | 0.61 |
| PDEL170-30 | 7.87 | 0.56 | 0.37 | 4.52 | 1.69 | 0.45 | 0.28 |
| PDEL170-60 | 7.41 | 0.11 | 0.33 | 5.33 | 0.54 | 0.86 | 0.24 |
| PDEL180-30 | 7.99 | 0.03 | 0.24 | 6.02 | 0.32 | 1.17 | 0.21 |
| Lcontrol | 2.80 | 0.84 | 0.06 | 0.11 | 1.32 | 0.20 | 0.27 |
| L170-30 | 2.95 | 0.74 | 0.03 | 0.31 | 1.26 | 0.39 | 0.22 |
| L170-60 | 2.50 | 0.67 | 0.25 | 0.25 | 0.65 | 0.51 | 0.17 |
| L180-30 | 2.19 | 0.43 | 0.27 | 0.27 | 0.23 | 0.93 | 0.06 |
| Conditions | Formic Acid | Acetic Acid | Lactic Acid | Furfural | HMF |
|---|---|---|---|---|---|
| 170-30 | 0.30 | 1.67 | 0.35 | 0.12 | 0.08 |
| 170-60 | 1.17 | 2.52 | 1.08 | 3.24 | 0.21 |
| 180-30 | 1.34 | 3.86 | 1.29 | 4.09 | 0.41 |
| Mw | Mn | Mw/Mn | |
|---|---|---|---|
| DEL | 6690 | 3800 | 1.76 |
| DEL170-30 | 4180 | 2290 | 1.83 |
| DEL170-60 | 4350 | 2720 | 1.60 |
| DEL180-30 | 3500 | 2300 | 1.52 |
| Lcontrol | 4200 | 2080 | 2.02 |
| L170-30 | 3870 | 1930 | 2.01 |
| L170-60 | 3450 | 1830 | 1.89 |
| L180-30 | 2290 | 1630 | 1.40 |
| β-O-4 | β-5 | β-β | FA2 | pCE2,6 | S/G b | |
|---|---|---|---|---|---|---|
| DEL | 49.96 a | 1.03 | 1.37 | 4.79 | 63.61 | 0.94 |
| PDEL170-30 | 33.03 | 0.44 | 0.22 | 4.08 | 59.07 | 1.33 |
| PDEL170-60 | 19.10 | 1.03 | 0.03 | 2.50 | 41.25 | 1.22 |
| PDEL180-30 | 12.06 | 1.33 | ND | 3.85 | 48.19 | 1.83 |
| Lcontrol | 15.66 | 3.31 | 0.78 | 3.31 | 42.60 | 1.54 |
| L170-30 | 12.88 | 3.62 | 0.88 | 3.17 | 31.68 | 1.30 |
| L170-60 | 8.92 | 3.93 | 0.36 | 2.57 | 24.04 | 1.61 |
| L180-30 | 2.69 | 2.49 | 0.23 | 1.63 | 18.74 | 1.26 |
| Sample | Aliphatic OH | Syringyl OH | Guaiacyl OH | p-Hydroxyphenyl OH c | Carboxylic Group | |
|---|---|---|---|---|---|---|
| C a | NC b | |||||
| DEL | 4.71 | 0.28 | 0.07 | 0.48 | 0.82 | 0.29 |
| PDEL170-30 | 3.27 | 0.52 | 0.11 | 0.50 | 0.83 | 0.30 |
| PDEL170-60 | 3.32 | 0.60 | 0.12 | 0.58 | 0.77 | 0.38 |
| PDEL180-30 | 3.80 | 0.58 | 0.12 | 0.58 | 0.77 | 0.39 |
| Lcontrol | 1.80 | 0.80 | 0.14 | 0.68 | 1.06 | 0.20 |
| L170-30 | 1.68 | 1.06 | 0.16 | 0.79 | 1.05 | 0.22 |
| L170-60 | 1.40 | 1.22 | 0.20 | 0.91 | 1.15 | 0.24 |
| L180-30 | 1.06 | 1.33 | 0.22 | 0.97 | 1.16 | 0.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Li, Z.; Zhang, X.; Min, D.; Wu, Y.; Wen, J.; Yuan, T. Effects of Hydrothermal Pretreatment on the Structural Characteristics of Organosolv Lignin from Triarrhena lutarioriparia. Polymers 2018, 10, 1157. https://doi.org/10.3390/polym10101157
Chen T, Li Z, Zhang X, Min D, Wu Y, Wen J, Yuan T. Effects of Hydrothermal Pretreatment on the Structural Characteristics of Organosolv Lignin from Triarrhena lutarioriparia. Polymers. 2018; 10(10):1157. https://doi.org/10.3390/polym10101157
Chicago/Turabian StyleChen, Tianying, Zhiwen Li, Xueming Zhang, Douyong Min, Yuying Wu, Jialong Wen, and Tongqi Yuan. 2018. "Effects of Hydrothermal Pretreatment on the Structural Characteristics of Organosolv Lignin from Triarrhena lutarioriparia" Polymers 10, no. 10: 1157. https://doi.org/10.3390/polym10101157