Constituents of Gastrodia elata and Their Neuroprotective Effects in HT22 Hippocampal Neuronal, R28 Retinal Cells, and BV2 Microglial Cells
Abstract
:1. Introduction
2. Results and Discussion
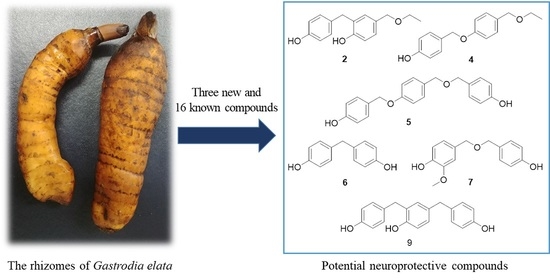
2.1. Isolation and Identification of Compounds from the Rhizomes of G. elata
2.2. Neuroprotective and NO Inhibitory Effects of Compounds from the Rhizomes of G. elata
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. 4-[[4-(4-Hydroxybenzyl)phenoxy]methyl]phenol (1)
3.3.2. 4-(Ethoxymethyl)-2-(4-hydroxybenzyl)phenol (2)
3.3.3. Ethyl S-(4-hydroxybenzyl) Glutathione (3)
3.4. Cell Culture
3.5. Sample Preparation
3.6. Cell Viability Assay
3.7. NO Production Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gooch, C.L.; Pracht, E.; Borenstein, A.R. The burden of neurological disease in the United States: A summary report and call to action. Ann. Neurol. 2017, 81, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Tiziani, S.; Park, G.; Kaul, M.; Paternostro, G. Cellular protection using Flt3 and PI3Kα inhibitors demonstrates multiple mechanisms of oxidative glutamate toxicity. Nat. Commun. 2014, 5, 3672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain—From eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44. [Google Scholar] [CrossRef]
- Kirkley, K.S.; Popichak, K.A.; Afzali, M.F.; Legare, M.E.; Tjalkens, R.B. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflamm. 2017, 14, 1–18. [Google Scholar] [CrossRef]
- Zhan, H.-D.; Zhou, H.-Y.; Sui, Y.-P.; Du, X.-L.; Wang, W.-h.; Dai, L.; Sui, F.; Huo, H.-R.; Jiang, T.-L. The rhizome of Gastrodia elata blume—An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef]
- Ojemann, L.M.; Nelson, W.L.; Shin, D.S.; Rowe, A.O.; Buchanan, R.A. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006, 8, 376–383. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, Y.W.; Kang, H.S.; Moon, H.; Sim, S.S.; Kim, C.J. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch. Pharm. Res. 2006, 29, 849–858. [Google Scholar] [CrossRef]
- Huang, Z.-B.; Wu, Z.; Chen, F.-K.; Zou, L.-B. The protective effects of phenolic constituents from Gastrodia elata on the cytotoxicity induced by KCl and glutamate. Arch. Pharm. Res. 2006, 29, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.Y.; Suh, S.I.; Lee, H.; Kim, I.S.; Kim, H.J.; Yoo, H.S.; Lee, S.R. Protective effects of several components of Gastrodia elata on lipid peroxidation in gerbil brain homogenates. Phytother. Res. 2007, 21, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Son, Y.; Kang, S.S.; Bae, C.-S.; Kim, J.-C.; Kim, S.-H.; Shin, T.; Moon, C. Neuropharmacological potential of Gastrodia elata Blume and its components. Evid. Based Complement. Alternat. Med. 2015, 2015, 1–14. [Google Scholar]
- Wang, Y.; Lin, S.; Chen, M.; Jiang, B.; Guo, Q.; Zhu, C.; Wang, S.; Yang, Y.; Shi, J. Chemical constituents from aqueous extract of Gastrodia elata. China J. Chin. Mater. Med. 2012, 37, 1775–1781. [Google Scholar]
- Tang, C.; Wang, L.; Liu, X.; Cheng, M.; Xiao, H. Chemical fingerprint and metabolic profile analysis of ethyl acetate fraction of Gastrodia elata by ultra performance liquid chromatography/quadrupole-time of flight mass spectrometry. J. Chromatogr. B 2016, 1011, 233–239. [Google Scholar] [CrossRef]
- Li, N.; Wang, K.-J.; Chen, J.-J.; Zhou, J. Phenolic compounds from the rhizomes of Gastrodia elata. J. Asian Nat. Prod. Res. 2007, 9, 373–377. [Google Scholar] [CrossRef]
- Han, A.R.; Ji Shin, H.; Rim Jeon, H.; Lee, J.H.; Lee, D.; Seo, E.K. Two new phenolic compounds from the rhizomes of Gastrodia elata Blume. Helv. Chim. Acta 2011, 94, 1310–1314. [Google Scholar] [CrossRef]
- Taguchi, H.; Yosioka, I.; Yamasaki, K.; Kim, I.H. Studies on the constituents of Gastrodia elata Blume. Chem. Pharm. Bull. 1981, 29, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-D.; Zhu, J.; Yang, R.; Liu, J.-P.; Li, L.; Zhang, H.-B. Phenolic constituents from the rhizomes of Gastrodia elata. Nat. Prod. Res. 2007, 21, 180–186. [Google Scholar] [CrossRef]
- Huang, Z.-B.; Song, D.-M.; Chen, F.-K. The chemical constituents isolated from Gastrodia elata BL. Chin. J. Med. Chem. 2005, 15, 227–229. [Google Scholar]
- Ciuffreda, P.; Casati, S.; Manzocchi, A. Complete 1H and 13C NMR spectral assignment of α-and β-adenosine, 2′-deoxyadenosine and their acetate derivatives. Magn. Reson. Chem. 2007, 45, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.-L.; Wang, Y.-N.; Zhu, C.-G.; Chen, M.-H.; Jiang, Z.-B.; Chen, N.-H.; Song, X.-Y.; Zhang, M.-J.; Shi, J.-G. 4-Hydroxybenzyl-substituted glutathione derivatives from Gastrodia elata. J. Asian Nat. Prod. Res. 2015, 17, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Shi, J.-M.; Gao, Y.-Q. Study on the volatile components of Gastrodia elata. J. Sichuan Norm. Univ. Nat. Sci. 2008, 1, 615–618. [Google Scholar]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seigel, G.M. R28 retinal precursor cells: The first 20 years. Mol. Vis. 2014, 20, 301. [Google Scholar] [PubMed]
- Boyce, J.A. Eicosanoids in asthma, allergic inflammation, and host defense. Curr. Mol. Med. 2008, 8, 335–349. [Google Scholar] [CrossRef]
- Yuan, L.; Qian, L.; Qian, Y.; Liu, J.; Yang, K.; Huang, Y.; Wang, C.; Li, Y.; Mu, X. Bisphenol F-induced nurotoxicity toward zebrafish embryos. Environ. Sci. Technol. 2019, 53, 14638–14648. [Google Scholar] [CrossRef]
- Kubo, T.; Maezawa, N.; Osada, M.; Katsumura, S.; Funae, Y.; Imaoka, S. Bisphenol A, an environmental endocrine-disrupting chemical, inhibits hypoxic response via degradation of hypoxia-inducible factor 1α (HIF-1α): Structural requirement of bisphenol A for degradation of HIF-1α. Biochem. Biophys. Res. Commun. 2004, 318, 1006–1011. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, H.; Hu, Y.; Li, J.; Li, Q. Gastrodin inhibits glutamate-induced apoptosis of PC12 cells via inhibition of CaMKII/ASK-1/p38 MAPK/p53 signaling cascade. Cell. Mol. Neurobiol. 2014, 34, 591–602. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Brasil, F.B.; Fürstenau, C.R. Nrf2 mediates the anti-apoptotic and anti-inflammatory effects induced by gastrodin in hydrogen peroxide–treated SH-SY5Y cells. J. Mol. Neurosci. 2019, 69, 115–122. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Bie, X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med. 2007, 73, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.-W.; Liu, Z.-Y.; Zhang, F.-L.; Zhang, L.; Li, F.; Liu, S.-Y.; He, J.-Y.; Xiao, Z.-C. Post-stroke gastrodin treatment ameliorates ischemic injury and increases neurogenesis and restores the Wnt/β-Catenin signaling in focal cerebral ischemia in mice. Brain Res. 2019, 1712, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-N.; Zong, Y.; Zhong, L.-M.; Li, Y.-M.; Zhang, W.; Bian, L.-G.; Ai, Q.-L. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS ONE 2011, 6, e21891. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-H.; Lee, D.-U.; Lee, J.-T.; Kim, J.-S.; Yong, C.-S.; Kim, J.-A.; Ha, J.-S. 4-Hydroxybenzaldehyde from Gastrodia elata B1. is active in the antioxidation and GABAergic neuromodulation of the rat brain. J. Ethnopharmacol. 2000, 73, 329–333. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ha, J.-H.; Yong, C.S.; Lee, D.-U.; Huh, K.; Kang, Y.S.; Lee, S.H.; Jung, M.-W.; Kim, J.-A. Inhibitory effects of constituents of Gastrodia elata Bl. on glutamate-induced apoptosis in IMR-32 human neuroblastoma cells. Arch. Pharm. Res. 1999, 22, 404–409. [Google Scholar] [CrossRef]
- Fukui, M.; Song, J.-H.; Choi, J.; Choi, H.J.; Zhu, B.T. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur. J. Pharmacol. 2009, 617, 1–11. [Google Scholar] [CrossRef]
- Kim, K.-A.; Kang, S.W.; Ahn, H.R.; Song, Y.; Yang, S.J.; Jung, S.H. Leaves of persimmon (Diospyros kaki Thunb.) ameliorate N-methyl-N-nitrosourea (MNU)-induced retinal degeneration in mice. J. Agric. Food Chem. 2015, 63, 7750–7759. [Google Scholar] [CrossRef]




| Position | 1 (CD3COCD3) | 2 (CDCl3) | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | 129.2 | 131.6 | ||
| 2 | 130.3 | 7.28, d (8.5) | 129.9 | 6.99, d (8.5) |
| 3 | 116.0 | 6.84, d (8.5) | 115.6 | 6.62, d (8.5) |
| 4 | 158.1 | 154.3 | ||
| 5 | 116.0 | 6.84, d (8.5) | 115.6 | 6.62, d (8.5) |
| 6 | 130.3 | 7.28, d (8.5) | 129.9 | 6.99, d (8.5) |
| 7 | 70.4 | 4.94, s | 35.6 | 4.41, s |
| 1′ | 135.1 | 130.2 | ||
| 2′ | 130.4 | 7.10, d (8.5) | 131.1 | 7.08, d (1.5) |
| 3′ | 115.6 | 6.89, d (8.5) | 127.6 | |
| 4′ | 158.2 | 153.7 | ||
| 5′ | 115.6 | 6.89, d (8.5) | 115.8 | 6.67, d (8.0) |
| 6′ | 130.4 | 7.10, d (8.5) | 128.0 | 7.06, dd (8.0, 1.5) |
| 7′ | 40.7 | 3.80, s | 72.8 | 3.85,s |
| 1″ | 133.5 | 65.9 | 3.57, q (7.0) | |
| 2″ | 130.5 | 7.02, d (8.0) | 15.3 | 1.24, t (7.0) |
| 3″ | 116.0 | 6.74, d (8.0) | ||
| 4″ | 156.5 | |||
| 5″ | 116.0 | 6.74, d (8.0) | ||
| 6″ | 130.5 | 7.02, d (8.0) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Kwon, J.; Lee, K.; Lee, J.W.; Jang, D.S.; Kwon, H.C. Constituents of Gastrodia elata and Their Neuroprotective Effects in HT22 Hippocampal Neuronal, R28 Retinal Cells, and BV2 Microglial Cells. Plants 2020, 9, 1051. https://doi.org/10.3390/plants9081051
Kim HM, Kwon J, Lee K, Lee JW, Jang DS, Kwon HC. Constituents of Gastrodia elata and Their Neuroprotective Effects in HT22 Hippocampal Neuronal, R28 Retinal Cells, and BV2 Microglial Cells. Plants. 2020; 9(8):1051. https://doi.org/10.3390/plants9081051
Chicago/Turabian StyleKim, Hye Mi, Jaeyoung Kwon, Kyerim Lee, Jae Wook Lee, Dae Sik Jang, and Hak Cheol Kwon. 2020. "Constituents of Gastrodia elata and Their Neuroprotective Effects in HT22 Hippocampal Neuronal, R28 Retinal Cells, and BV2 Microglial Cells" Plants 9, no. 8: 1051. https://doi.org/10.3390/plants9081051