Arabidopsis CCoAOMT1 Plays a Role in Drought Stress Response via ROS- and ABA-Dependent Manners
Abstract
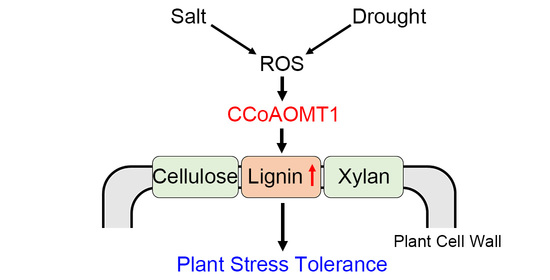
:1. Introduction
2. Results
2.1. Arabidopsis CCoAOMT1 Is Involved in the Abiotic Stress Response
2.2. Loss of CCoAOMT1 Function Exhibits a Sensitive Phenotype to Drought Stress by Accumulating a Higher Level of H2O2
2.3. CCoAOMT1 Functions in Drought Response by Modulating Both ABA-Dependent and ABA-Independent Pathways
2.4. CCoAOMT1 Gene Is Responsive to ABA Stress
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Stress Conditions
4.2. Quantitative Real-Time PCR (qRT-PCR) Analyses
4.3. Water Loss Assay
4.4. Chlorophyll Measurement
4.5. H2O2 Staining and Its Content Measurement
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gull, A.; Ahmad Lone, A.; Ul Islam Wani, N. Biotic and abiotic stresses in plants. Abiotic Biot. Stress Plants 2019, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bechtold, U.; Field, B. Molecular mechanisms controlling plant growth during abiotic stress. J. Exp. Bot. 2018, 69, 2753–2758. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of abiotic stress on crops. Sustain. Crop. Prod. 2020, 1–21. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants (Basel) 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Hamann, T. The plant cell wall integrity maintenance mechanism-Concepts for organization and mode of action. Plant Cell Physiol. 2015, 56, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Lee, B.R.; Kim, K.Y.; Jung, W.J.; Avice, J.C.; Ourry, A.; Kim, T.H. Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.). J. Exp. Bot. 2007, 58, 1271–1279. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, C.C.; Guo, W.D.; Li, X.B.; Lu, M.; Yu, C.L. Differential expression of cell wall related genes in the elongation zone of rice roots under water deficit. Russ. J. Plant Physiol. 2006, 53, 390–395. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Cruz De Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Cerny, M.; Habanova, H.; Berka, M.; Luklova, M.; Brzobohaty, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Chan, Z. Ros regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasulu, N.; Harshavardhan, V.T.; Govind, G.; Seiler, C.; Kohli, A. Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 2012, 506, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Wani, S.H.; Bhattacharjee, S.; Burritt, D.J.; Tran, L.S.P. Drought stress tolerance in plants, vol 2: Molecular and genetic perspectives. Drought Stress Toler. Plants Vol 2 Mol. Genet. Perspect. 2016, 1–587. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Paul, S.; Basu, S. Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep. 2013, 32, 985–1006. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Zhang, K.; An, H.; Hu, K.; Wen, J.; Shen, J.; Ma, C.; Yi, B.; Tu, J.; et al. Comparative analysis of the Brassica napus root and leaf transcript profiling in response to drought stress. Int. J. Mol. Sci. 2015, 16, 18752–18777. [Google Scholar] [CrossRef] [Green Version]
- Fellenberg, C.; Van Ohlen, M. The role of CCoAOMT1 and COMT1 in Arabidopsis anthers. Planta 2012, 236, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Do, C.T.; Pollet, B.; Thévenin, J.; Sibout, R.; Denoue, D.; Barrière, Y.; Lapierre, C.; Jouanin, L. Both caffeoyl Coenzyme A 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta 2007, 226, 1117–1129. [Google Scholar] [CrossRef]
- Chun, H.J.; Baek, D.; Cho, H.M.; Lee, S.H.; Jin, B.J.; Yun, D.J.; Hong, Y.S.; Kim, M.C. Lignin biosynthesis genes play critical roles in the adaptation of Arabidopsis plants to high-salt stress. Plant Signal. Behav. 2019, 14, 1–4. [Google Scholar] [CrossRef]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.E.; Shabala, S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J. Exp. Bot. 2011, 62, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T. Regulation of drought tolerance by gene manipulation of 9- cis -epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, J.; Zhang, J.; Hao, L.; Hua, J.; Duan, L.; Zhang, M.; Li, Z. Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions. Plant Biotechnol. J. 2013, 11, 747–758. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A. Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. Omics. J. Integr. Biol. 2011, 15, 763–774. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14, 15–46. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, K.; Masuda, A.; Kuwano, M.; Yokota, A.; Akashi, K. Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant Cell Physiol. 2008, 49, 226–241. [Google Scholar] [CrossRef] [Green Version]
- Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Oxidative stress in plants. Antioxidants 2020, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, A.; Zhang, J.; Jiang, M. Abscisic acid is a key inducer of hydrogen peroxide production in leaves of maize plants exposed to water stress. Plant Cell Physiol. 2006, 47, 1484–1495. [Google Scholar] [CrossRef] [Green Version]
- Arve, L.E.; Carvalho, D.R.A.; Olsen, J.E.; Torre, S. ABA induces H2O2 production in guard cells, but does not close the stomata on vicia faba leaves developed at high air humidity. Plant Signal. Behav. 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Aubert, Y.; Vile, D.; Pervent, M.; Aldon, D.; Ranty, B.; Simonneau, T.; Vavasseur, A.; Galaud, J.P. RD20, a stress-inducible caleosin, participates in stomatal control, transpiration and drought tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 1975–1987. [Google Scholar] [CrossRef] [Green Version]
- Blée, E.; Boachon, B.; Burcklen, M.; Le Gaé, M.; Abdulsamie, H.; Heintz, D.; Ehlting, J.; Herrfurth, C.; Feussner, I.; Bessoule, J.J. The reductase activity of the arabidopsis caleosin RESPONSIVE TO DESSICATION20 mediates gibberellin-dependent flowering time, abscisic acid sensitivity, and tolerance to oxidative stress1[w]. Plant Physiol. 2014, 166, 109–124. [Google Scholar] [CrossRef] [Green Version]
- del Rodríguez-Gacio, M.C.; Matilla-Vázquez, M.A.; Matilla, A.J. Seed dormancy and ABA signaling: The breakthrough goes on. Plant Signal. Behav. 2009, 4, 1035–1048. [Google Scholar] [CrossRef] [Green Version]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Saha, U. The effect of drought on lignin content and digestibility of Tifton-85 and coastal bermudagrass (Cynodon dactylon L.) Hays Produced in Georgia. Int. J. Appl. Agric. Sci. 2016, 2, 69. [Google Scholar] [CrossRef]
- Pereira, L.; Domingues-Junior, A.P.; Jansen, S.; Choat, B.; Mazzafera, P. Is embolism resistance in plant xylem associated with quantity and characteristics of lignin? Trees Struct. Funct. 2018, 32, 349–358. [Google Scholar] [CrossRef]
- Taylor-Teeples, M.; Lin, L.; De Lucas, M.; Turco, G.; Toal, T.W.; Gaudinier, A.; Young, N.F.; Trabucco, G.M.; Veling, M.T.; Lamothe, R.; et al. An arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 2015, 517, 571–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, S.; Iwai, Y.; Fukuda, H. Cargo-dependent and cell wall-associated xylem transport in Arabidopsis. New Phytol. 2019, 222, 159–170. [Google Scholar] [CrossRef]
- Faragó, D.; Sass, L.; Valkai, I.; Andrási, N.; Szabados, L. Plantsize offers an affordable, non-destructive method to measure plant size and color in vitro. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudi, A.; O’Brien, J.A. Detection of hydrogen peroxide by DAB staining in arabidopsis leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef] [Green Version]
- Grellet Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, H.J.; Lim, L.H.; Cheong, M.S.; Baek, D.; Park, M.S.; Cho, H.M.; Lee, S.H.; Jin, B.J.; No, D.H.; Cha, Y.J.; et al. Arabidopsis CCoAOMT1 Plays a Role in Drought Stress Response via ROS- and ABA-Dependent Manners. Plants 2021, 10, 831. https://doi.org/10.3390/plants10050831
Chun HJ, Lim LH, Cheong MS, Baek D, Park MS, Cho HM, Lee SH, Jin BJ, No DH, Cha YJ, et al. Arabidopsis CCoAOMT1 Plays a Role in Drought Stress Response via ROS- and ABA-Dependent Manners. Plants. 2021; 10(5):831. https://doi.org/10.3390/plants10050831
Chicago/Turabian StyleChun, Hyun Jin, Lack Hyeon Lim, Mi Sun Cheong, Dongwon Baek, Mi Suk Park, Hyun Min Cho, Su Hyeon Lee, Byung Jun Jin, Dong Hyeon No, Ye Jin Cha, and et al. 2021. "Arabidopsis CCoAOMT1 Plays a Role in Drought Stress Response via ROS- and ABA-Dependent Manners" Plants 10, no. 5: 831. https://doi.org/10.3390/plants10050831