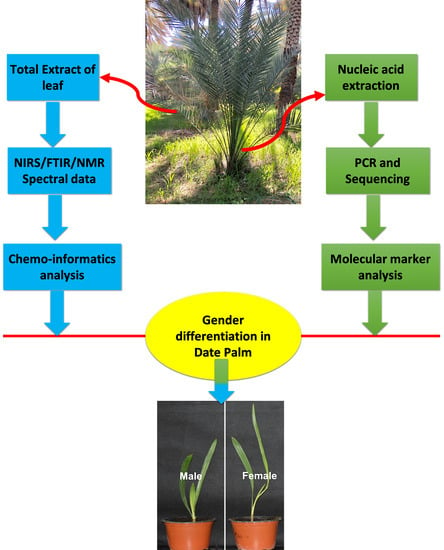
Spectroscopic and Molecular Methods to Differentiate Gender in Immature Date Palm (Phoenix dactylifera L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. NIRS Based Discrimination in Sex of Date Palm
2.2. FT-IR Based Discrimination Date Palm Sex
2.3. NMR Based Validation of Sex Differentiation in Date Palms
2.4. Molecular Marker Analysis of Sex-Specific Traits in Date Palms
3. Materials and Methods
3.1. Plant Collection and Sampling
3.2. Spectral Analysis using NIRS, FTIR, and NMR
3.3. Multivariate Data Analysis
3.4. PCR, RT-PCR, and qPCR-Based Analyses of Selected Genes/Molecular Markers
3.5. Genetic Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlesworth, B.; Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 1978, 112, 975–997. [Google Scholar] [CrossRef]
- Kersten, B.; Pakull, B.; Fladung, M. Genomics of sex determination in dioecious trees and woody plants. Trees 2017, 31, 1113–1125. [Google Scholar] [CrossRef]
- Dhawan, C.; Kharb, P.; Sharma, R.; Uppal, S.; Aggarwal, R.K. Development of male-specific SCAR marker in date palm (Phoenix dactylifera L.). Tree Genet. Genomes 2013, 9, 1143–1150. [Google Scholar] [CrossRef]
- Lemaitre, C.; Braga, M.D.V.; Gautier, C.; Sagot, M.-F.; Tannier, E.; Marais, G.A.B. Footprints of inversions at present and past pseudoautosomal boundaries in human sex chromosomes. Genome Biol. Evol. 2009, 1, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlesworth, D.; Charlesworth, B.; Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 2005, 95, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terauchi, R.; Kahl, G. Mapping of the Dioscorea tokoro genome: AFLP markers linked to sex. Genome 1999, 42, 752–762. [Google Scholar] [CrossRef]
- Parasnis, A.S.; Ramakrishna, W.; Chowdari, K.V.; Gupta, V.S.; Ranjekar, P.K. Microsatellite (GATA)n reveals sex-specific differences in Papaya. Theor. Appl. Genet. 1999, 99, 1047–1052. [Google Scholar] [CrossRef]
- Spada, A.; Caporali, E.; Marziani, G.; Portaluppi, P.; Restivo, F.M.; Tassi, F.; Falavigna, A. A genetic map of Asparagus officinalis based on integrated RFLP, RAPD and AFLP molecular markers. Theor. Appl. Genet. 1998, 97, 1083–1089. [Google Scholar] [CrossRef]
- Daher, A. Cell cycle arrest characterizes the transition from a bisexual floral bud to a unisexual flower in Phoenix dactylifera. Ann. Bot. 2010, 106, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Billotte, N.; Marseillac, N.; Brottier, P.; Noyer, J.-L.; Jacquemoud-Collet, J.-P.; Moreau, C.; Couvreur, T.; Chevallier, M.-H.; Pintaud, J.-C.; Risterucci, A.-M. Nuclear microsatellite markers for the date palm (Phoenix dactylifera L.): Characterization and utility across the genus Phoenix and in other palm genera. Mol. Ecol. Resour. 2004, 4, 256–258. [Google Scholar] [CrossRef]
- Younis, R.A.; Ismail, O.M.; Soliman, S.S. Identification of sex-specific DNA markers for date palm (Phoenix dactylifera L.) using RAPD and ISSR techniques. Res. J. Agric. Biol. Sci. 2008, 4, 278–284. [Google Scholar]
- Al-Dous, E.K.; George, B.; E Al-Mahmoud, M.; Al-Jaber, M.Y.; Wang, H.; Salameh, Y.M.; Al-Azwani, E.K.; Chaluvadi, S.R.; Pontaroli, A.C.; DeBarry, J.; et al. De novo genome sequencing and comparative genomics of date palm (Phoenix dactylifera). Nat. Biotechnol. 2011, 29, 521–527. [Google Scholar] [CrossRef]
- Yaish, M.W.; Kumar, P.P. Salt tolerance research in date palm tree (Phoenix dactylifera L.), past, present, and future perspectives. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekheet, S.A.; Hanafy, M.S. Towards sex determination of date palm. In Date Palm Biotechnology; Springer: New York, NY, USA, 2011. [Google Scholar]
- Al-Mahmoud, M.E.; Al-Dous, E.K.; Al-Azwani, E.K.; Malek, J.A. DNA-based assays to distinguish date palm (Arecaceae) gender. Am. J. Bot. 2012, 99, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Elmeer, K.; Mattat, I. Marker-assisted sex differentiation in date palm using simple sequence repeats. 3 Biotech 2012, 2, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Williams, R.; Prakash, C.S.; He, G. Identification and characterization of gene-based SSR markers in date palm (Phoenix dactylifera L.). BMC Plant Biol. 2012, 12, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehdi-Azouzi, S.; Cherif, E.; Guenni, K.; Ben Abdelkrim, A.; Bermil, A.; Rhouma, S.; Ben Salah, M.; Santoni, S.; Pintaud, J.C.; Aberlenc-Bertossi, F.; et al. Endemic insular and coastal Tunisian date palm genetic diversity. Genetica 2016, 144, 181–190. [Google Scholar] [CrossRef] [PubMed]
- El-Yazal, S.S.; Alharby, H.; El-Yazal, M.S.; Hassan, G.; Rady, M. Molecular identification of some seedling of date palm (Phoenix dactylifera L.) Males’ trees. J. Anim. Plant Sci. 2017, 27, 1287–1294. [Google Scholar]
- Cherif, E.; Zehdi, S.; Castillo, K.; Chabrillange, N.; Abdoulkader, S.; Pintaud, J.-C.; Santoni, S.; Salhi-Hannachi, A.; Glémin, S.; Aberlenc-Bertossi, F. Male-specific DNA markers provide genetic evidence of an XY chromosome system, a recombination arrest and allow the tracing of paternal lineages in date palm. New Phytol. 2013, 197, 409–415. [Google Scholar] [CrossRef]
- Cao, D.; Lutz, A.; Hill, C.B.; Callahan, D.L.; Roessner, U. A quantitative profiling method of phytohormones and other metabolites applied to barley roots subjected to salinity stress. Front. Plant Sci. 2017, 7, 2070. [Google Scholar] [CrossRef] [Green Version]
- Marden, J.H.; Mangan, S.A.; Peterson, M.P.; Wafula, E.; Fescemyer, H.W.; Der, J.P.; Depamphilis, C.W.; Comita, L.S. Ecological genomics of tropical trees: How local population size and allelic diversity of resistance genes relate to immune responses, susceptibility to pathogens, and negative density dependence. Mol. Ecol. 2017, 26, 2498–2513. [Google Scholar] [CrossRef]
- Cozzolino, D. Near infrared spectroscopy in natural products analysis. Planta Med. 2009, 75, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Warton, D.I.; Blanchet, F.G.; O’Hara, R.B.; Ovaskainen, O.; Taskinen, S.; Walker, S.C.; Hui, F.K. So many variables: Joint modeling in community ecology. Trends Ecol. Evol. 2015, 30, 766–779. [Google Scholar] [CrossRef]
- Chan, K.L.; Ho, C.L.; Namasivayam, P.; Napis, S. A simple and rapid method for RNA isolation from plant tissues with high phenolic compounds and polysaccharides. Nat. Protocol 2007, 184. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ali, L.; Al-Harrasi, A.; Mabood, F.; Al-Broumi, M.; Khan, A.L.; Hussain, H.; Hussain, J.; Csuk, R. Quantification of AKBA in Boswellia sacra Using NIRS Coupled with PLSR as an Alternative Method and Cross-Validation by HPLC. Phytochem. Anal. 2018, 29, 137–143. [Google Scholar] [CrossRef]
- Agelet, L.E.; Hurburgh, C.R. Limitations and current applications of Near Infrared Spectroscopy for single seed analysis. Talanta 2014, 121, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Min, T.G.; Kang, W.S. Nondestructive separation of viable and nonviable gourd (Lagenaria siceraria) seeds using single seed near infrared spectroscopy. Hortic. Environ. Biotechnol. 2003, 44, 545–548. [Google Scholar]
- Olesen, M.H.; Carstensen, J.M.; Boelt, B. Multispectral imaging as a potential tool for seed health testing of spinach (Spinacia oleracea L.). Seed Sci. Technol. 2011, 39, 140–150. [Google Scholar] [CrossRef]
- Wang, L.; Lee, F.S.; Wang, X.; He, Y. Feasibility study of quantifying and discriminating soybean oil adulteration in camellia oils by attenuated total reflectance MIR and fiber optic diffuse reflectance NIR. Food Chem. 2006, 95, 529–536. [Google Scholar] [CrossRef]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). Wiley Interdisciplinary Reviews. Comput. Stat 2010, 2, 97–106. [Google Scholar]
- Tigabu, M.; Fjellström, J.; Odén, P.C.; Teketay, D. Germination of Juniperus procera seeds in response to stratification and smoke treatments, and detection of insect-damaged seeds with VIS+ NIR spectroscopy. New For. 2007, 33, 155–169. [Google Scholar] [CrossRef]
- Lang, C.; Costa, F.R.C.; Camargo, J.L.C.; Durgante, F.M.; Vicentini, A. Near infrared spectroscopy facilitates rapid identification of both young and mature Amazonian tree species. PLoS ONE 2015, 10, e0134521. [Google Scholar] [CrossRef]
- Carrascal, L.M.; Galván, I.; Gordo, O. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 2009, 118, 681–690. [Google Scholar] [CrossRef]
- Ohsowski, B.M.; Dunfield, K.E.; Klironomos, J.N.; Hart, M.M. Improving plant biomass estimation in the field using partial least squares regression and ridge regression. Botany 2016, 94, 501–508. [Google Scholar] [CrossRef]
- Tormena, C.D.; Pauli, E.D.; Marcheafave, G.G.; Scheel, G.L.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S. FT-IR biomarkers of sexual dimorphism in yerba-mate plants: Seasonal and light accessibility effects. Microchem. J. 2020, 158, 105329. [Google Scholar] [CrossRef]
- Gurdeniz, G.; Ozen, B. Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data. Food Chem. 2009, 116, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Martelo-Vidal, M.J.; Vázquez, M. Determination of polyphenolic compounds of red wines by UV–VIS–NIR spectroscopy and chemometrics tools. Food Chem. 2014, 158, 28–34. [Google Scholar] [CrossRef]
- Zheng, H.; Yde, C.C.; Arnberg, K.; Mølgaard, C.; Michaelsen, K.F.; Larnkjær, A.; Bertram, H.C. NMR-based metabolomic profiling of overweight adolescents: An elucidation of the effects of inter-/intraindividual differences, gender, and pubertal development. BioMed Res. Int. 2014, 2014, 537157. [Google Scholar] [CrossRef]
- Das, K.; Ganie, S.H.; Mangla, Y.; Dar, T.-U.-H.; Chaudhary, M.; Thakur, R.K.; Raina, S.N.; Goel, S.; Tandon, R. ISSR markers for gender identification and genetic diagnosis of Hippophae rhamnoides ssp. turkestanica growing at high altitudes in Ladakh region (Jammu and Kashmir). Protoplasma 2017, 254, 1063–1077. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Resour. 2006, 6, 288–295. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.L.; Al-Harrasi, A.; Numan, M.; AbdulKareem, N.M.; Mabood, F.; Al-Rawahi, A. Spectroscopic and Molecular Methods to Differentiate Gender in Immature Date Palm (Phoenix dactylifera L.). Plants 2021, 10, 536. https://doi.org/10.3390/plants10030536
Khan AL, Al-Harrasi A, Numan M, AbdulKareem NM, Mabood F, Al-Rawahi A. Spectroscopic and Molecular Methods to Differentiate Gender in Immature Date Palm (Phoenix dactylifera L.). Plants. 2021; 10(3):536. https://doi.org/10.3390/plants10030536
Chicago/Turabian StyleKhan, Abdul Latif, Ahmed Al-Harrasi, Muhammad Numan, Noor Mazin AbdulKareem, Fazal Mabood, and Ahmed Al-Rawahi. 2021. "Spectroscopic and Molecular Methods to Differentiate Gender in Immature Date Palm (Phoenix dactylifera L.)" Plants 10, no. 3: 536. https://doi.org/10.3390/plants10030536