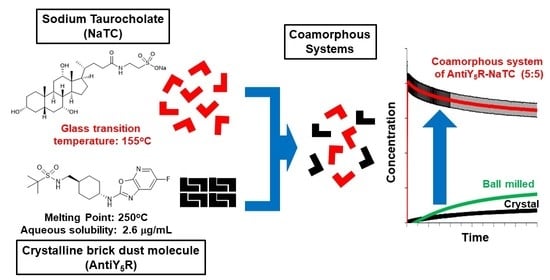
Formation of a Stable Co-Amorphous System for a Brick Dust Molecule by Utilizing Sodium Taurocholate with High Glass Transition Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetry/Differential Thermal Analysis
2.3. Solubility
2.4. Preparation of Physical Mixtures of AntiY5R and Sodium Taurocholate
2.5. Preparation of Co-Amorphous Systems of AntiY5R and Sodium Taurocholate
2.6. X-ray Powder Diffraction
2.7. Differential Scanning Calorimetry (DSC)
2.8. Calculation of Theoretical Glass Transition Temperature
2.9. Measurement of True Densities
2.10. Fourier-Transform Infrared Spectroscopy
2.11. In Vitro Dissolution Test
2.12. Isothermal Crystallization
3. Results and Discussion
3.1. Physicochemical Properties of Pure Components
3.2. Glass Transition Behaviors of AntiY5R and Its Co-Amorphous Systems
3.3. Preparation of the Co-Amorphous Systems
3.4. Physical Stability of the Co-Amorphous Systems
3.5. Fourier-Transformed Infrared Spectra of the Co-Amorphous Systems
3.6. Dissolution Studies of Co-Amorphous Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Griesenauer, R.H.; Kinch, M.S. 2016 in review: FDA approvals of new molecular entities. Drug Discov. Today 2017, 22, 1593–1597. [Google Scholar] [CrossRef]
- Han, J.; Wei, Y.; Lu, Y.; Wang, R.; Zhang, J.; Gao, Y.; Qian, S. Co-amorphous systems for the delivery of poorly water-soluble drugs: Recent advances and an update. Expert Opin. Drug Deliv. 2020, 17, 1411–1435. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Sahbaz, Y.; Williams, H.D.; Nguyen, T.H.; Saunders, J.; Ford, L.; Charman, S.A.; Scammells, P.J.; Porter, C.J. Transformation of poorly water-soluble drugs into lipophilic ionic liquids enhances oral drug exposure from lipid based formulations. Mol. Pharm. 2015, 12, 1980–1991. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Charman, W.N.; Porter, C.J.H. Computational prediction of formulation strategies for beyond-rule-of-5 compounds. Adv. Drug Deliv. Rev. 2016, 101, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.S.; Miller, W.K.; Pluntze, A.M.; Stewart, A.M.; Cape, J.L.; Grass, M.E.; Morgen, M.M. Acetic Acid as Processing Aid Dramatically Improves Organic Solvent Solubility of Weakly Basic Drugs for Spray Dried Dispersion Manufacture. Pharmaceutics 2022, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Koehl, N.J.; Holm, R.; Kuentz, M.; Griffin, B.T. New Insights into Using Lipid Based Suspensions for ‘Brick Dust’ Molecules: Case Study of Nilotinib. Pharm. Res. 2019, 36, 56. [Google Scholar] [CrossRef]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Commercially bioavailable proprietary technologies and their marketed products. Drug Discov. Today 2013, 18, 936–949. [Google Scholar] [CrossRef]
- Stoler, E.; Warner, J.C. Non-Covalent Derivatives: Cocrystals and Eutectics. Molecules 2015, 20, 14833–14848. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Rohani, S.; Gong, J.; Wang, J. Recent Developments in the Crystallization Process: Toward the Pharmaceutical Industry. Engineering 2017, 3, 343–353. [Google Scholar] [CrossRef]
- Ngilirabanga, J.B.; Samsodien, H. Pharmaceutical co-crystal: An alternative strategy forenhanced physicochemical properties and drug synergy. Nano Select 2021, 2, 512–526. [Google Scholar] [CrossRef]
- Vo, C.L.; Park, C.; Lee, B.J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A.; Alruwaili, N.K.; Alotaibi, F.; Alanazi, A.; Shakeel, F. Potential of solid dispersions to enhance solubility, bioavailability, and therapeutic efficacy of poorly water-soluble drugs: Newer formulation techniques, current marketed scenario and patents. Drug Deliv. 2020, 27, 1625–1643. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O'Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, K.; Harada, T.; Miura, K.; Yoshihashi, Y.; Yonemochi, E.; Terada, K.; Moriyama, H. Relationship between crystallization tendencies during cooling from melt and isothermal storage: Toward a general understanding of physical stability of pharmaceutical glasses. Mol. Pharm. 2014, 11, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Tang, X.; Taylor, L.S. Investigating the Correlation between Miscibility and Physical Stability of Amorphous Solid Dispersions Using Fluorescence-Based Techniques. Mol. Pharm. 2016, 13, 3988–4000. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, Y.; Ueda, K.; Okada, H.; Takeda, J.; Karashima, M.; Yazawa, K.; Higashi, K.; Kawakami, K.; Ikeda, Y.; Moribe, K. Effect of Drug–Polymer Interactions through Hypromellose Acetate Succinate Substituents on the Physical Stability on Solid Dispersions Studied by Fourier-Transform Infrared and Solid-State Nuclear Magnetic Resonance. Mol. Pharm. 2019, 16, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Okada, H.; Zhao, Z.; Higashi, K.; Moribe, K. Application of solid-state 13C relaxation time to prediction of the recrystallization inhibition strength of polymers on amorphous felodipine at low polymer loading. Int. J. Pharm. 2020, 581, 119300. [Google Scholar] [CrossRef]
- Li, N.; Cape, J.L.; Mankani, B.R.; Zemlyanov, D.Y.; Shepard, K.B.; Morgen, M.M.; Taylor, L.S. Water-Induced Phase Separation of Spray-Dried Amorphous Solid Dispersions. Mol. Pharm. 2020, 17, 4004–4017. [Google Scholar] [CrossRef]
- Luebbert, C.; Sadowski, G. Moisture-induced phase separation and recrystallization in amorphous solid dispersions. Int. J. Pharm. 2017, 532, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug. Deliv. Sci. Technol. 2020, 55, 101459. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.F.; Pinto, R.M.A.; Simões, S. Hot-melt extrusion in the pharmaceutical industry: Toward filing a new drug application. Drug Discov. Today 2019, 24, 1749–1768. [Google Scholar] [CrossRef]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef]
- Mota, R.; Portugal, C.M.; Lino, P.; Silva, R.C.; Ricardo, A.A.; Vicente, J. Leveraging Hollow Fiber Membranes and High Boiling Point Organic Solvents in the Production of Enteric Amorphous Solid Dispersions. In Proceedings of the 2020 Virtual AIChE Annual Meeting, Virtual Meeting, 16–20 November 2020. [Google Scholar]
- Al-Obaidi, H.; Lawrence, M.J.; Al-Saden, N.; Ke, P. Investigation of griseofulvin and hydroxypropylmethyl cellulose acetate succinate miscibility in ball milled solid dispersions. Int. J. Pharm. 2013, 443, 95–102. [Google Scholar] [CrossRef]
- Liu, J.; Grohganz, H.; Löbmann, K.; Rades, T.; Hempel, N.J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics 2021, 13, 389. [Google Scholar] [CrossRef]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Sarabu, S.; Bandari, S.; Kallakunta, V.R.; Tiwari, R.; Patil, H.; Repka, M.A. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: Part II. Expert Opin. Drug Deliv. 2019, 16, 567–582. [Google Scholar] [CrossRef]
- Kasten, G.; Löbmann, K.; Grohganz, H.; Rades, T. Co-former selection for co-amorphous drug-amino acid formulations. Int. J. Pharm. 2019, 557, 366–373. [Google Scholar] [CrossRef]
- Wostry, M.; Plappert, H.; Grohganz, H. Preparation of Co-Amorphous Systems by Freeze-Drying. Pharmaceutics 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Duarte, Í.; Paisana, M.; Löbmann, K.; Rades, T.; Grohganz, H. Process Optimization and Upscaling of Spray-Dried Drug-Amino acid Co-Amorphous Formulations. Pharmaceutics 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs--Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85, 873–881. [Google Scholar] [CrossRef]
- Ueda, H.; Wu, W.; Löbmann, K.; Grohganz, H.; Müllertz, A.; Rades, T. Application of a Salt Coformer in a Co-Amorphous Drug System Dramatically Enhances the Glass Transition Temperature: A Case Study of the Ternary System Carbamazepine, Citric Acid, and l-Arginine. Mol. Pharm. 2018, 15, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Mattern, M.; Winter, G.; Kohnert, U.; Lee, G. Formulation of proteins in vacuum-dried glasses. II. Process and storage stability in sugar-free amino acid systems. Pharm. Dev. Technol. 1999, 4, 199–208. [Google Scholar] [CrossRef]
- Evgenyi, Y.S.; Larry, A.G. Formulation and Process Development Strategies for Manufacturing Biopharmaceuticals; Jameel, F., Hershenson, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 507–509. [Google Scholar]
- Ueda, H.; Hirakawa, Y.; Miyano, T.; Imono, M.; Tse, J.Y.; Uchiyama, H.; Tozuka, Y.; Kadota, K. Design of a Stable Coamorphous System Using Lactose as an Antiplasticizing Agent for Diphenhydramine Hydrochloride with a Low Glass Transition Temperature. Mol. Pharm. 2022, 19, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Lalge, R.; Kaur, N.; Duggirala, N.K.; Suryanarayanan, R. Dual Functionality of Bile Acid: Physical Stabilization of Drugs in the Amorphous Form and Solubility Enhancement in Solution. Mol. Pharm. 2022, 19, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Gniado, K.; Löbmann, K.; Rades, T.; Erxleben, A. The influence of co-formers on the dissolution rates of co-amorphous sulfamerazine/excipient systems. Int. J. Pharm. 2016, 504, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Gniado, K.; MacFhionnghaile, P.; McArdle, P.; Erxleben, A. The natural bile acid surfactant sodium taurocholate (NaTC) as a coformer in coamorphous systems: Enhanced physical stability and dissolution behavior of coamorphous drug-NaTc systems. Int. J. Pharm. 2018, 535, 132–139. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. ICH Q1A (R2) Stability Testing of New Drug Substances and Drug Products. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products (accessed on 20 November 2022).
- Wu, W.; Löbmann, K.; Rades, T.; Grohganz, H. On the role of salt formation and structural similarity of co-formers in co-amorphous drug delivery systems. Int. J. Pharm. 2018, 535, 86–94. [Google Scholar] [CrossRef]
- Ueda, H.; Wakabayashi, S.; Kikuchi, J.; Ida, Y.; Kadota, K.; Tozuka, Y. Anomalous role change of tertiary amino and ester groups as hydrogen acceptors in eudragit E based solid dispersion depending on the concentration of naproxen. Mol. Pharm. 2015, 12, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Aikawa, S.; Kashima, Y.; Kikuchi, J.; Ida, Y.; Tanino, T.; Kadota, K.; Tozuka, Y. Anti-plasticizing effect of amorphous indomethacin induced by specific intermolecular interactions with PVA copolymer. J. Pharm. Sci. 2014, 103, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Pokorný, V.; Štejfa, V.; Havlín, J.; Růžička, K.; Fulem, M. Heat Capacities of l-Histidine, l-Phenylalanine, l-Proline, l-Tryptophan and l-Tyrosine. Molecules 2021, 26, 4298. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kadota, K.; Imono, M.; Ito, T.; Kunita, A.; Tozuka, Y. Co-amorphous Formation Induced by Combination of Tranilast and Diphenhydramine Hydrochloride. J. Pharm. Sci. 2017, 106, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newa, M.; Bhandari, K.H.; Kim, J.O.; Im, J.S.; Kim, J.A.; Yoo, B.K.; Woo, J.S.; Choi, H.G.; Yong, C.S. Enhancement of solubility, dissolution and bioavailability of ibuprofen in solid dispersion systems. Chem. Pharm. Bull. 2008, 56, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, M.F.; Nogueira, B.A.; Tabanez, A.M.; Fausto, R.; Pinto, R.M.A.; Simões, S. Enhanced solid-state stability of amorphous ibrutinib formulations prepared by hot-melt extrusion. Int. J. Pharm. 2020, 579, 119156. [Google Scholar] [CrossRef]
- Ueda, H.; Hirakawa, Y.; Tanaka, H.; Miyano, T.; Sugita, K. Applicability of an Experimental Grade of Hydroxypropyl Methylcellulose Acetate Succinate as a Carrier for Formation of Solid Dispersion with Indomethacin. Pharmaceutics 2021, 13, 353. [Google Scholar] [CrossRef]
- Benbow, N.L.; Rozenberga, L.; McQuillan, A.J.; Krasowska, M.; Beattie, D.A. ATR FTIR Study of the Interaction of TiO2 Nanoparticle Films with β-Lactoglobulin and Bile Salts. Langmuir 2021, 37, 13278–13290. [Google Scholar] [CrossRef]
- Vinod, K.S.; Periandy, S.; Govindarajan, M. Spectroscopic [FT-IR and FT-Raman] and molecular modeling (MM) study of benzene sulfonamide molecule using quantum chemical calculations. J. Mol. Struc. 2016, 1116, 226–235. [Google Scholar] [CrossRef]
- Srivastav, G.; Yadav, B.; Yadav, R.K.; Yadav, R.A. Vibrational spectra and molecular structure of sulfanilamide: IR and Low temperature Raman studies and DFT investigation of monomeric and dimeric forms. Vib. Spectrosc. 2021, 112, 103199. [Google Scholar] [CrossRef]
- Singh, V.B.; Singh, A.K.; Rai, A.K.; Singh, A.N.; Rai, D.K. Vibrational assignment of the normal modes of 2-phenyl-4-(4-methoxy benzylidene)-2-oxazolin-5-one via FTIR and Raman spectra, and ab inito calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 687–693. [Google Scholar] [CrossRef]
- Bernal, H.G.; Caero, L.C.; Finocchio, E.; Busca, G. An FT-IR study of the adsorption and reactivity of tert-butyl hydroperoxide over oxide catalysts. Appl. Catal. A 2009, 369, 27–35. [Google Scholar] [CrossRef]
- Boopalachandran, P.; Laane, J. Vibrational spectra, structure, and theoretical calculations of 2-fluoro- and 3-fluoropyridine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1191–1195. [Google Scholar] [CrossRef]
- Xiao-Hong, L.; Zheng-Xin, T.; Xian-Zhou, Z. Molecular structure, IR spectra of 2-mercaptobenzothiazole and 2-mercaptobenzoxazole by density functional theory and ab initio Hartree-Fock calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 168–173. [Google Scholar] [CrossRef]
- Nugrahani, I.; Fisandra, F.; Horikawa, A.; Uekusa, H. New Sodium Mefenamate–Nicotinamide Multicomponent Crystal Development to Modulate Solubility and Dissolution: Preparation, Structural, and Performance Study. J. Pharm. Sci. 2021, 110, 3246–3260. [Google Scholar] [CrossRef]
- Kumar, S.; Nanda, A. Pharmaceutical Cocrystals: An Overview. Indian J. Pharm. Sci. 2017, 79, 858–871. [Google Scholar] [CrossRef]
- Vinarov, Z.; Katev, V.; Burdzhiev, N.; Tcholakova, S.; Denkov, N. Effect of Surfactant-Bile Interactions on the Solubility of Hydrophobic Drugs in Biorelevant Dissolution Media. Mol. Pharm. 2018, 15, 5741–5753. [Google Scholar] [CrossRef]









| Compounds | Tm (°C) | Tdeg (°C) |
|---|---|---|
| AntiY5R | 250.2 ± 0.2 | >300.5 ± 1.1 |
| NaTC | N.D. | >306.3 ± 4.1 |
| Compounds | Tm (°C) | Tg (°C) | Crystallization (°C) |
|---|---|---|---|
| AntiY5R | 250.6 ± 1.2 | 69.5 ± 0.4 1 | ca 170 |
| NaTC | N.D. | 155.2 ± 0.6 2 | N.D. |
| Sampling Time | Crystalline (µg/mL) | Ball-Milled Crystalline (µg/mL) | AntiY5R-NaTC (Molar Ratio) (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| 5:5 | 4:6 | 3:7 | 2:8 | 1:9 | |||
| Within 2 min 1 | Not applicable | Not applicable | 20.5 ± 1.4 | 17.7 ± 1.5 | 16.3 ± 0.8 | 14.8 ± 2.3 | 12.3 ± 6.2 |
| 180 min | 1.7 ± 0.1 | 4.8 ± 0.1 | 16.4 ± 1.1 | 15.1 ± 1.4 | 9.8 ± 1.0 | 2.1 ± 0.5 | 2.1 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aikawa, S.; Tanaka, H.; Ueda, H.; Maruyama, M.; Higaki, K. Formation of a Stable Co-Amorphous System for a Brick Dust Molecule by Utilizing Sodium Taurocholate with High Glass Transition Temperature. Pharmaceutics 2023, 15, 84. https://doi.org/10.3390/pharmaceutics15010084
Aikawa S, Tanaka H, Ueda H, Maruyama M, Higaki K. Formation of a Stable Co-Amorphous System for a Brick Dust Molecule by Utilizing Sodium Taurocholate with High Glass Transition Temperature. Pharmaceutics. 2023; 15(1):84. https://doi.org/10.3390/pharmaceutics15010084
Chicago/Turabian StyleAikawa, Shohei, Hironori Tanaka, Hiroshi Ueda, Masato Maruyama, and Kazutaka Higaki. 2023. "Formation of a Stable Co-Amorphous System for a Brick Dust Molecule by Utilizing Sodium Taurocholate with High Glass Transition Temperature" Pharmaceutics 15, no. 1: 84. https://doi.org/10.3390/pharmaceutics15010084