A Fully Human Monoclonal Antibody Targeting cKIT Is a Potent Inhibitor of Pathological Choroidal Neovascularization in Mice
Abstract
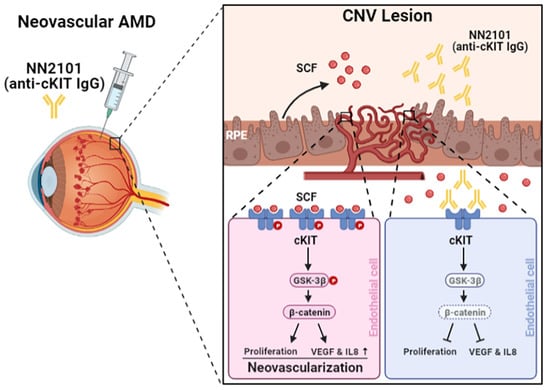
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blotting
2.3. In Vitro Angiogenesis Assay
2.4. Animals
2.5. Animal Model of Laser-Induced CNV
2.6. Combination Index (CI)
2.7. Immunohistochemistry and Terminal Deoxynucleotidyl Transferase dUTP Nick End-Labeling (TUNEL) Assay
2.8. Live and Dead Cell Double Staining Assay
2.9. Ocular Pharmacokinetics (PK)
2.10. Retinal Vascular Development
2.11. Statistical Analysis
3. Results
3.1. The Anti-cKIT Blocking IgG, NN2101, Inhibits SCF/cKIT-Mediated Angiogenic Signaling and Angiogenesis in Human Endothelial Cells at Hypoxia
3.2. Intravitreal Administration of NN2101 Suppresses SCF/cKIT Signaling and the Pathological CNV in a Murine Model of Neovascular AMD
3.3. A Combination Therapy of NN2101 and Aflibercept Additively Inhibits Pathological CNV in Mice
3.4. NN2101 Does Not Induce Ocular Toxicity
3.5. NN2101 Does Not Affect Early Retinal Vascular Development in Mice
3.6. PK of Intravitreal NN2101 in the Rabbit Eyes and Serum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Grisanti, S.; Tatar, O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog. Retin. Eye Res. 2008, 27, 372–390. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holz, F.G.; Schmitz-Valckenberg, S.; Fleckenstein, M. Recent developments in the treatment of age-related macular degeneration. J. Clin. Investig. 2014, 124, 1430–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhisitkul, R.B.; Mendes, T.S.; Rofagha, S.; Enanoria, W.; Boyer, D.S.; Sadda, S.R.; Zhang, K. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: The SEVEN-UP study. Am. J. Ophthalmol. 2015, 159, 915–924.e912. [Google Scholar] [CrossRef]
- Ford, K.M.; Saint-Geniez, M.; Walshe, T.; Zahr, A.; D’Amore, P.A. Expression and role of VEGF in the adult retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9478–9487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunwald, J.E.; Pistilli, M.; Daniel, E.; Ying, G.S.; Pan, W.; Jaffe, G.J.; Toth, C.A.; Hagstrom, S.A.; Maguire, M.G.; Martin, D.F.; et al. Incidence and Growth of Geographic Atrophy during 5 Years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2017, 124, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, T.; Westenskow, P.D.; Bravo, S.; Aguilar, E.; Friedlander, M. Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Investig. 2012, 122, 4213–4217. [Google Scholar] [CrossRef] [Green Version]
- Sadda, S.R.; Tuomi, L.L.; Ding, B.; Fung, A.E.; Hopkins, J.J. Macular Atrophy in the HARBOR Study for Neovascular Age-Related Macular Degeneration. Ophthalmology 2018, 125, 878–886. [Google Scholar] [CrossRef] [Green Version]
- Spitzer, M.S.; Wallenfels-Thilo, B.; Sierra, A.; Yoeruek, E.; Peters, S.; Henke-Fahle, S.; Bartz-Schmidt, K.U.; Szurman, P.; Tuebingen Bevacizumab Study, G. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br. J. Ophthalmol. 2006, 90, 1316–1321. [Google Scholar] [CrossRef] [Green Version]
- Usui-Ouchi, A.; Friedlander, M. Anti-VEGF therapy: Higher potency and long-lasting antagonism are not necessarily better. J. Clin. Investig. 2019, 129, 3032–3034. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.L.; Seo, S.; Kim, J.T.; Kim, J.; Kim, W.; Yeo, Y.; Sung, J.H.; Park, S.G.; Suh, W. SCF (Stem Cell Factor) and cKIT Modulate Pathological Ocular Neovascularization. Arter. Thromb. Vasc. Biol. 2019, 39, 2120–2131. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Kangave, D.; Mairaj Siddiquei, M.; Geboes, K. Angiogenic and vasculogenic factors in the vitreous from patients with proliferative diabetic retinopathy. J. Diabetes Res. 2013, 2013, 539658. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Struyf, S.; Opdenakker, G.; Van Damme, J.; Geboes, K. Expression of stem cell factor/c-kit signaling pathway components in diabetic fibrovascular epiretinal membranes. Mol. Vis. 2010, 16, 1098–1107. [Google Scholar] [CrossRef]
- Kim, J.O.; Kim, H.N.; Kim, K.H.; Baek, E.J.; Park, J.Y.; Ha, K.; Heo, D.R.; Seo, M.D.; Park, S.G. Development and characterization of a fully human antibody targeting SCF/c-kit signaling. Int. J. Biol. Macromol. 2020, 159, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.L.; Suh, W. Apatinib, an Inhibitor of Vascular Endothelial Growth Factor Receptor 2, Suppresses Pathologic Ocular Neovascularization in Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3592–3599. [Google Scholar] [CrossRef] [Green Version]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.L.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharm. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Park, S.J.; Choi, Y.; Na, Y.M.; Hong, H.K.; Park, J.Y.; Park, K.H.; Chung, J.Y.; Woo, S.J. Intraocular Pharmacokinetics of Intravitreal Aflibercept (Eylea) in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2612–2617. [Google Scholar] [CrossRef]
- Meyer, C.H.; Krohne, T.U.; Charbel Issa, P.; Liu, Z.; Holz, F.G. Routes for Drug Delivery to the Eye and Retina: Intravitreal Injections. Dev. Ophthalmol. 2016, 55, 63–70. [Google Scholar] [CrossRef]
- Varela-Fernandez, R.; Diaz-Tome, V.; Luaces-Rodriguez, A.; Conde-Penedo, A.; Garcia-Otero, X.; Luzardo-Alvarez, A.; Fernandez-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christoforidis, J.B.; Williams, M.M.; Kothandaraman, S.; Kumar, K.; Epitropoulos, F.J.; Knopp, M.V. Pharmacokinetic properties of intravitreal I-124-aflibercept in a rabbit model using PET/CT. Curr. Eye Res. 2012, 37, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quintanilla, L.; Luaces-Rodriguez, A.; Gil-Martinez, M.; Mondelo-Garcia, C.; Maronas, O.; Mangas-Sanjuan, V.; Gonzalez-Barcia, M.; Zarra-Ferro, I.; Aguiar, P.; Otero-Espinar, F.J.; et al. Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 365. [Google Scholar] [CrossRef] [Green Version]
- Broudy, V.C. Stem cell factor and hematopoiesis. Blood 1997, 90, 1345–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sette, C.; Dolci, S.; Geremia, R.; Rossi, P. The role of stem cell factor and of alternative c-kit gene products in the establishment, maintenance and function of germ cells. Int. J. Dev. Biol. 2000, 44, 599–608. [Google Scholar] [PubMed]
- Yoshida, H.; Kunisada, T.; Grimm, T.; Nishimura, E.K.; Nishioka, E.; Nishikawa, S.I. Review: Melanocyte migration and survival controlled by SCF/c-kit expression. J. Investig. Dermatol. Symp. Proc. 2001, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]







| PK Parameters | Vitreous | Aqueous | Retina/Choroid | Serum |
|---|---|---|---|---|
| T1/2, h | 103.3 | 203.0 | 128.9 | |
| MRT, h | 131.3 | 208.5 | 243.7 | 311.6 |
| V/F, mL | 1.3 | 12.0 | 98.1 | |
| CL/F, mL/h | 0.009 | 0.041 | 0.527 | |
| Cmax, μg/mL | 378.3 | 100.8 | 3.2 | 0.3 |
| AUC, h × μg/mL | 63,921.0 | 12,771.9 | 1101.6 | 132.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.; Kim, K.L.; Yeo, Y.; Kim, R.-I.; Jeong, H.; Kim, J.-O.; Song, S.-H.; An, M.-J.; Kim, J.-W.; Hong, H.K.; et al. A Fully Human Monoclonal Antibody Targeting cKIT Is a Potent Inhibitor of Pathological Choroidal Neovascularization in Mice. Pharmaceutics 2021, 13, 1308. https://doi.org/10.3390/pharmaceutics13081308
Seo S, Kim KL, Yeo Y, Kim R-I, Jeong H, Kim J-O, Song S-H, An M-J, Kim J-W, Hong HK, et al. A Fully Human Monoclonal Antibody Targeting cKIT Is a Potent Inhibitor of Pathological Choroidal Neovascularization in Mice. Pharmaceutics. 2021; 13(8):1308. https://doi.org/10.3390/pharmaceutics13081308
Chicago/Turabian StyleSeo, Songyi, Koung Li Kim, Yeongju Yeo, Ryul-I Kim, Hayoung Jeong, Jin-Ock Kim, Sun-Hwa Song, Mi-Jin An, Jung-Woong Kim, Hye Kyoung Hong, and et al. 2021. "A Fully Human Monoclonal Antibody Targeting cKIT Is a Potent Inhibitor of Pathological Choroidal Neovascularization in Mice" Pharmaceutics 13, no. 8: 1308. https://doi.org/10.3390/pharmaceutics13081308