Self-Nanoemulsion Loaded with a Combination of Isotretinoin, an Anti-Acne Drug, and Quercetin: Preparation, Optimization, and In Vivo Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
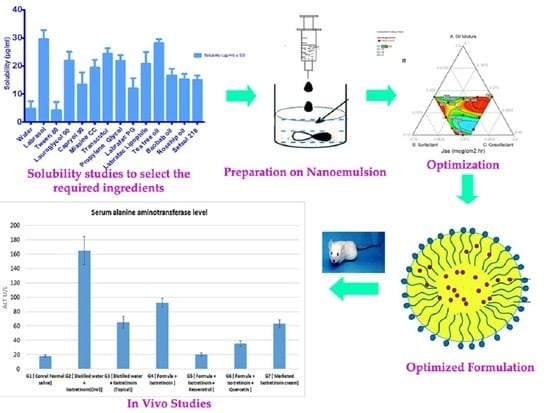
2.2.1. Determination of ITT Solubility
2.2.2. Pre-Formulation Studies
2.2.3. Mixture Design for the Development of ITT-QRS-Loaded SNEDDS
2.2.4. Preparation of ITT-QRS Nanoemulsion
2.2.5. Characterization of ITT-QRS Nanoemulsion
Measurement of Globule Size
Ex Vivo Permeation Studies to Evaluate the Jss of ITT from Each Formula
Inhibition Zone against Staphylococcus aureus
2.2.6. Optimization of ISN Formulations
2.2.7. Preparation and Evaluation of the Optimum Formula According to the Mixture Design
2.2.8. In Vitro Release Studies
2.2.9. Animal Studies
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. ITT Solubility Studies
3.2. Pre-Formulation Studies
3.3. Optimization of ITT-Loaded SNEDDS
3.4. Validation of Experimental Design
3.5. In Vitro Permeation/Release Study
3.6. Ex Vivo Permeation Study
3.7. In Vivo Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanlayavattanakul, M.; Lourith, N. Therapeutic agents and herbs in topical application for acne treat-ment. Int. J. Cosmet. Sci. 2011, 33, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.A.; Khunger, N. A morphological study of acne scarring and its relationship between se-verity and treatment of active acne. J. Cutan. Aesthet. Surg. 2020, 13, 210–216. [Google Scholar] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Esmael, A.; Hassan, M.G.; Amer, M.M.; Abdelrahman, S.; Hamed, A.M.; Abd-Raboh, H.A.; Han, H. Antimicrobial activity of certain natural-based plant oils against the antibiotic-resistant acne bacteria. Saudi J. Biol. Sci. 2020, 27, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Bahmani, M.; Shahinfard, N.; Nafchi, A.M.; Saberianpour, S.; Rafieian-Kopaei, M. Medicinal Plants for the Treatment of Acne Vulgaris: A Review of Recent Evidences. Jundishapur J. Microbiol. 2015, 8, e25580. [Google Scholar] [CrossRef] [Green Version]
- Totté, J.E.E.; Van Der Feltz, W.T.; Hennekam, M.; van Belkum, A.; Van Zuuren, E.J.; Pasmans, S.G.M.A. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: A systematic review and me-ta-analysis. Br. J. Dermatol. 2016, 175, 687–695. [Google Scholar] [CrossRef]
- Oge’, L.K.; Broussard, A.; Marshall, M.D. Acne Vulgaris: Diagnosis and Treatment. Am. Fam. Phys. 2019, 100, 475–484. [Google Scholar]
- Gollnick, H.; Zouboulis, C.C. Not All Acne Is Acne Vulgaris. Dtsch. Aerzteblatt Online 2014, 111, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, N.I.; Cohen, P.R. Acne Vulgaris: A Patient and Physician’s Experience. Dermatol. Ther. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A review of diagnosis and treatment of acne in adult female pa-tients. Int. J. Womens Dermatol. 2018, 4, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; James, W.D. Staphylococcus aureus Infection as a Complication of Isotretinoin Therapy. Arch. Dermatol. 1987, 123, 5–7. [Google Scholar] [CrossRef]
- Ahmadv, H.; Javanbakht, A.M.A.; Pour, H.M. M Effects of oral isotretinoin on serum lipids and gamma glutamyl transpeptidase activity in acne vulgaris patients. Afr. J. Pharm. Pharmacol. 2011, 5, 1338–1341. [Google Scholar] [CrossRef] [Green Version]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic compounds from humulus lupulus as natural antimicrobial products: New weapons in the fight against methicillin resistant Staphylococcus aureus, leishmania mexicana and trypa-nosoma brucei strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zhang, H.; Wang, Y.; Song, F.; Yuan, Y. Inhibitory effects of quercetin on the progression of liver fibrosis through the regulation of NF-кB/IкBα, p38 MAPK, and Bcl-2/Bax signaling. Int. Immunopharmacol. 2017, 47, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Knezevic, A.H.; Šver, L.; Terzić, S.; Basic, I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharmacol. 2004, 94, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. The Role of Quercetin, Flavonols and Flavones in Modulating Inflammatory Cell Function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; De Risi, C. Research Progress in the Modification of Quercetin Leading to Anticancer Agents. Molecules 2017, 22, 1270. [Google Scholar] [CrossRef]
- Aguirre, L.; Arias, N.; Teresa Macarulla, M.; Gracia, A.; Portillo, M.P. Beneficial effects of quercetin on obesi-ty and diabetes. Open Nutraceuticals J. 2011, 4, 189–198. [Google Scholar]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal nanocarriers for enhanced skin delivery of quercetin with functions of an-ti-tyrosinase and antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, P.; Choi, D.K. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int. J. Nanomed. 2016, 11, 1987–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for poorly wa-ter-soluble talinolol: Preparation, in vitroand in vivoAssessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izham, M.N.M.; Hussin, Y.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Rasedee, A.; Alitheen, N.B. Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials 2019, 9, 1028. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioa-vailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: Design, formulation, pharmacokinetic and bioavailability eval-uation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef]
- Dou, Y.X.; Zhou, J.T.; Wang, T.T.; Huang, Y.F.; Chen, V.P.; Xie, Y.L.; Lin, Z.X.; Gao, J.S.; Su, Z.R.; Zeng, H.F. Self-nanoemulsifying drug delivery system of bruceine D: A new approach for an-ti-ulcerative colitis. Int. J. Nanomed. 2018, 13, 5887–5907. [Google Scholar] [CrossRef] [Green Version]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formula-tion insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Filho, P.A.D.R. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef]
- Jang, M.H.; Piao, X.L.; Kim, J.M.; Kwon, S.W.; Park, J.H. Inhibition of cholinesterase and amyloid-&bgr; aggregation by resveratrol oligomers from Vitis amurensis. Phyther. Res. 2008, 22, 544–549. [Google Scholar]
- De Santana, F.B.; Gontijo, L.C.; Mitsutake, H.; Mazivila, S.J.; De Souza, L.M.; Neto, W.B. Non-destructive fraud detection in rosehip oil by MIR spectroscopy and chemometrics. Food Chem. 2016, 209, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Figueroa, M.; Blanco-Méndez, J. Transdermal delivery of methotrexate: Iontophoretic delivery from hydrogels and passive delivery from microemulsions. Int. J. Pharm. 2001, 215, 57–65. [Google Scholar] [CrossRef]
- Djekic, L.; Primorac, M. The influence of cosurfactants and oils on the formation of pharmaceutical micro-emulsions based on PEG-8 caprylic/capric glycerides. Int. J. Pharm. 2008, 352, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Ahmad, O.A.A.; Fahmy, U.A.; Alkhalidi, H.M. Nanovesicular systems loaded with a recently approved second generation type-5 phospodiesterase inhibitor (avanafil): I. Plackett-Burman screening and characterization. J. Drug Deliv. Sci. Technol. 2018, 43, 154–159. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Rohaninasab, M.; Behrangi, E.; Nahad, Z.M.; Rasi, A. Efficacy of fixed daily 20 mg of isotretinoin in moderate to severe scar prone acne. Adv. Biomed. Res. 2014, 3, 103. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Hosny, K.M. Development and optimization of ocular in situ gels loaded with ciprofloxacin cubic liquid crystalline nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101710. [Google Scholar] [CrossRef]
- Hosny, K.M. Nanosized Cubosomal Thermogelling Dispersion Loaded with Saquinavir Mesylate to Improve Its Bioavailability: Preparation, Optimization, in vitro and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 5113–5129. [Google Scholar] [CrossRef]
- Shao, M.; Hussain, Z.; Thu, H.E.; Khan, S.; Katas, H.; Ahmed, T.A.; Tripathy, M.; Leng, J.; Qin, H.L.; Bukhari, S.N.A. Drug nanocarrier, the future of atopic diseases: Ad-vanced drug delivery systems and smart management of disease. Coll. Surf. B Biointerfaces 2016, 147, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Rizg, W.Y. Quality by design approach to optimize the formula-tion variables influencing the characteristics of biodegradable intramuscular in-situ gel loaded with alen-dronate sodium for osteoporosis. PLoS ONE 2018, 13, e0197540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.; Li, C.; Liu, M.; Qiu, Q.; Luo, X.; Liu, X.; Hu, L.; Deng, Y.; Song, Y. Effect of Kupffer cells depletion on ABC phenomenon induced by Kupffer cells-targeted liposomes. Asian J. Pharm. Sci. 2019, 14, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.A.; Abd El-Alim, S.H. Vesicular Nanocarriers: A Potential Platform for Dermal and Transdermal Drug Delivery. Nanopharm. Princ. Appl. 2020, 2, 155–209. [Google Scholar]
- Hanifah, M.; Jufri, M. Formulation and Stability Testing of Nanoemulsion Lotion Containing Centella asiatica Extract. J. Young Pharm. 2018, 10, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Gaba, B.; Fazil, M.; Khan, S.; Ali, A.; Baboota, S.; Ali, J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Pashirova, T.N.; Lukashenko, S.S.; Zakharov, S.V.; Voloshina, A.D.; Zhiltsova, E.P.; Zobov, V.V.; Souto, E.B.; Zakharova, L.Y. Self-assembling systems based on quaternized derivatives of 1,4-diazabicyclo[2.2.2]octane in nutrient broth as antimicrobial agents and carriers for hydrophobic drugs. Coll. Surf. B Biointerfaces 2015, 127, 266–273. [Google Scholar] [CrossRef]
- Sharma, V.; Chaudhary, U. An overview on indigenous knowledge of Achyranthes aspera. J. Crit. Rev. 2015, 2, 7–19. [Google Scholar]
- Gupta, C.; Vikram, A.; Tripathi, D.N.; RamaRao, P.; Jena, G. Antioxidant and antimutagenic effect of quercetin against DEN induced hepatotoxicity in rat. Phytother. Res. 2009, 24, 119–128. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Cano-Martínez, A.; Díaz-Díaz, E.; Manzano-Pech, L.; Gamas-Magaña, A.; Castrejón-Tellez, V.; Tapia-Cortina, C.; Pérez-Torres, I. Resveratrol and quercetin administration improves an-tioxidant defenses and reduces fatty liver in metabolic syndrome rats. Molecules 2019, 24, 1297. [Google Scholar] [CrossRef] [Green Version]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural Antioxidant Control of Neuropathic Pain—Exploring the Role of Mito-chondrial SIRT3 Pathway. Antioxidants 2020, 9, 1103. [Google Scholar] [CrossRef]
- Lee, Y.H.; Scharnitz, T.P.; Muscat, J.; Chen, A.; Gupta-Elera, G.; Kirby, J.S. Laboratory monitoring during isotretinoin therapy for acne: A systematic review and meta-analysis. JAMA Dermatol. 2016, 152, 35–44. [Google Scholar] [CrossRef]
- Goforoushan, F.; Azimi, H.; Goldust, M. Efficacy of Vitamin E to Prevent Dermal Complications of Isotretinoin. Pak. J. Biol. Sci. 2013, 16, 548–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Formula | Formulation |
|---|---|
| 1 | Rosehip oil (0.2 g) + Lauroglycol-90 (0.6 g) + Propylene glycol (0.2 g) + 20 mg ITT+ 20 mg QRS |
| 2 | Rosehip oil (0.2 g) + Lauroglycol-90 (0.6 g) + Propylene glycol (0.2 g) + 40 mg ITT+ 40 mg QRS |
| 3 | Rosehip oil (0.2 g) + Lauroglycol-90 (0.6 g) + Propylene glycol (0.2 g) + 60 mg ITT+ 60 mg QRS |
| 4 | Rosehip oil (0.3 g) + Lauroglycol-90 (0.6 g) + Propylene glycol (0.1 g) + 20 mg ITT+ 20 mg QRS |
| 5 | Rosehip oil (0.3 g) + Lauroglycol-90 (0.4 g) + Propylene glycol (0.3 g) + 20 mg ITT+ 20 mg QRS |
| 6 | Rosehip oil (0.1 g) + Lauroglycol-90 (0.7 g) + Propylene glycol (0.2 g) + 20 mg ITT+ 20 mg QRS |
| 7 | Rosehip oil (0.1 g) + Lauroglycol-90 (0.5 g) + Propylene glycol (0.4 g) + 20 mg ITT+ 20 mg QRS |
| 8 | Rosehip oil (0.2 g) + Lauroglycol-90 (0.6 g) + Propylene glycol (0.2 g) + 20 mg ITT+ 20 mg QRS |
| 9 | Rosehip oil (0.3 g) + Lauroglycol-90 (0.6 g) + Propylene glycol (0.1 g) + 20 mg ITT+ 20 mg QRS |
| 10 | Rosehip oil (0.1 g) + Lauroglycol-90 (0.5 g) + Propylene glycol (0.4 g) + 20 mg ITT+ 20 mg QRS |
| Component | Level | Response | Constraints | |
|---|---|---|---|---|
| Low | High | |||
| Rosehip oil (%), (X1) | 10 | 30 | Mean globule size (Y1) | Minimum |
| Lauroglycol-90 (%), (X2) | 40 | 60 | Steady-state inflow (Jss) (Y2) | Maximum |
| Propylene glycol (%), (X3) | 10 | 30 | Inhibition zone (Y3) | Maximum |
| Run | Independent Variables | ||
|---|---|---|---|
| (X1): Oil (Rosehip Oil) | (X2): Surfactant (Lauroglycol-90) | (X3): Cosurfactant (Propylene Glycol) | |
| 1 | 0.3 | 0.5 | 0.2 |
| 2 | 0.2 | 0.5 | 0.3 |
| 3 | 0.3 | 0.533333 | 0.166667 |
| 4 | 0.2 | 0.6 | 0.2 |
| 5 | 0.2 | 0.6 | 0.2 |
| 6 | 0.233333 | 0.533333 | 0.233333 |
| 7 | 0.166667 | 0.566667 | 0.266667 |
| 8 | 0.2 | 0.5 | 0.3 |
| 9 | 0.3 | 0.6 | 0.1 |
| 10 | 0.1 | 0.6 | 0.3 |
| 11 | 0.2 | 0.5 | 0.3 |
| 12 | 0.2 | 0.6 | 0.2 |
| 13 | 0.266667 | 0.466667 | 0.266667 |
| 14 | 0.1 | 0.6 | 0.3 |
| 15 | 0.3 | 0.466667 | 0.233333 |
| 16 | 0.3 | 0.4 | 0.3 |
| Formula | Amount of ITT (mg) | Particle Size ± SD (nm) |
|---|---|---|
| 1 | 20 | 114.11 ± 13.28 |
| 2 | 40 | 286.22 ± 15.62 |
| 3 | 60 | 350.4 ± 21.32 |
| 4 | 20 | 311.21 ± 12.33 |
| 5 | 20 | 297.22 ± 11.11 |
| 6 | 20 | 91.12 ± 6.75 |
| 7 | 20 | 81.33 ± 2.76 |
| 8 | 20 | 130.21 ± 12.3 |
| 9 | 20 | 350.55 ± 16.1 |
| 10 | 20 | 100.34 ± 7.80 |
| Run | Globule Size * (nm) | Jss * (µg/cm2/h) | Inhibition Zone * (mm) |
|---|---|---|---|
| 1 | 370 ± 13 | 115 ± 9 | 25 ± 4 |
| 2 | 220 ± 11 | 240 ± 13 | 9 ± 2 |
| 3 | 355 ± 14 | 95 ± 7 | 26 ± 6 |
| 4 | 200 ± 10 | 130 ± 9 | 11 ± 2 |
| 5 | 199 ± 10 | 128 ± 6 | 12 ± 4 |
| 6 | 265 ± 13 | 135 ± 11 | 15 ± 2 |
| 7 | 167 ± 14 | 205 ± 14 | 6 ± 2 |
| 8 | 218 ± 12 | 238 ± 12 | 8 ± 3 |
| 9 | 340 ± 16 | 107 ± 10 | 27 ± 5 |
| 10 | 130 ± 9 | 270 ± 17 | 3 ± 1 |
| 11 | 217 ± 13 | 235 ± 23 | 9 ± 4 |
| 12 | 203 ± 12 | 127 ± 11 | 11 ± 2 |
| 13 | 300 ± 14 | 177 ± 16 | 19 ± 4 |
| 14 | 132 ± 17 | 268 ± 12 | 3 ± 1 |
| 15 | 381 ± 12 | 140 ± 15 | 24 ± 5 |
| 16 | 390 ± 18 | 220 ± 18 | 23 ± 2 |
| Source | Globule Size | Jss | Inhibition Zone | |||
|---|---|---|---|---|---|---|
| Adjusted R2 | Predicted R2 | Adjusted R2 | Predicted R2 | Adjusted R2 | Predicted R2 | |
| Linear | 0.9455 | 0.9217 | 0.8463 | 0.7051 | 0.9420 | 0.9160 |
| Quadratic | 0.9933 | 0.9896 | 0.9943 | 0.9887 | 0.9948 | 0.9937 |
| Special Cubic | 0.9926 | 0.9783 | 0.9950 | 0.9695 | 0.9950 | 0.9881 |
| Cubic | 0.9996 | 0.9989 | 0.9968 | |||
| Term | Responses | |||||
|---|---|---|---|---|---|---|
| Globule Size | Jss | Inhibition Zone | ||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Model | 4645.30 | <0.0001 | 928.75 | <0.0001 | 574.28 | <0.0001 |
| Linear Mixture | 19,919.44 | <0.0001 | 3223.03 | <0.0001 | 1368.24 | <0.0001 |
| AB | 74.69 | 0.0001 | 18.73 | 0.0034 | 74.79 | <0.0001 |
| AC | 30.07 | 0.0015 | 42.96 | 0.0003 | 55.22 | <0.0001 |
| BC | 1.30 | 0.2975 | 29.73 | 0.0010 | 0.1197 | 0.7365 |
| ABC | 30.87 | 0.0014 | 0.0041 | 0.9510 | --- | --- |
| AB (A to B) | 154.29 | <0.0001 | 6.63 | 0.0367 | --- | --- |
| AC (A to C) | 165.73 | <0.0001 | 20.28 | 0.0028 | --- | --- |
| BC (B to C) | 13.88 | 0.0098 | 928.75 | <0.0001 | --- | --- |
| Number. | Parameter | Predicted Values | Experimental Values | Relative Error (%) |
|---|---|---|---|---|
| 1. | Globule size (nm) | 249 | 245 | 1.6 |
| 2. | Jss (µg/cm2/h) | 222.05 | 228 | –2.4 |
| 3. | Inhibition zone (mm) | 15 | 14.5 | 0.4 |
| Groups | AST | ALT | MDA | GSH |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| G1 [Control normal saline] | 19.8 ± 1.89 | 21.85 ± 1.47 | 0.682 ± 0.029 | 636.47 ± 16.56 |
| G2 [Distilled water + ITT (Oral)] | 38.5 ± 3.26 | 46.57 ± 4.54 | 1.278 ± 0.030 | 383.71 ± 13.8 |
| G3 [Distilled water + ITT (Topical)] | 31.5 ± 3.50 | 38.225 ± 3.76 | 0.984 ± 0.021 | 445.09 ± 17.48 |
| G4 [Optimum ITT-QRS SNEDDS] | 20.75 ± 2.61 | 22.035 ± 2.11 | 0.674 ± 0.033 | 660.30 ± 22.08 |
| G5 [Optimum ITT SNEDDS without QRS] | 29.17 ± 2.62 | 36.895 ± 2.76 | 1024 ± 0.021 | 413.63 ± 25.76 |
| G6 [Optimum ITT SNEDDS without ITT] | 19.16 ± 2.09 | 22.735 ± 1.58 | 0.696 ± 0.019 | 697.26 ± 22.08 |
| G7 [Marketed ITT cream] | 34.41 ± 3.46 | 38.26 ± 2.07 | 0.956 ± 0.025 | 495.89 ± 19.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosny, K.M.; Al Nahyah, K.S.; Alhakamy, N.A. Self-Nanoemulsion Loaded with a Combination of Isotretinoin, an Anti-Acne Drug, and Quercetin: Preparation, Optimization, and In Vivo Assessment. Pharmaceutics 2021, 13, 46. https://doi.org/10.3390/pharmaceutics13010046
Hosny KM, Al Nahyah KS, Alhakamy NA. Self-Nanoemulsion Loaded with a Combination of Isotretinoin, an Anti-Acne Drug, and Quercetin: Preparation, Optimization, and In Vivo Assessment. Pharmaceutics. 2021; 13(1):46. https://doi.org/10.3390/pharmaceutics13010046
Chicago/Turabian StyleHosny, Khaled M., Khalid S. Al Nahyah, and Nabil A. Alhakamy. 2021. "Self-Nanoemulsion Loaded with a Combination of Isotretinoin, an Anti-Acne Drug, and Quercetin: Preparation, Optimization, and In Vivo Assessment" Pharmaceutics 13, no. 1: 46. https://doi.org/10.3390/pharmaceutics13010046