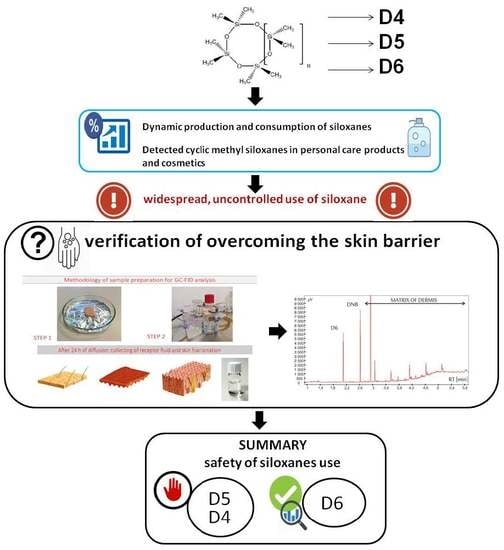
Ex Vivo Human Skin is not a Barrier for Cyclic Siloxanes (Cyclic Silicones): Evidence of Diffusion, Bioaccumulation, and Risk of Dermal Absorption Using a New Validated GC-FID Procedure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
 –O(CH2CH2O)8CHCH2OH; CAS Number 9002-93-1; HLB = 13) (Sigma-Aldrich, Steinheim, Germany); sodium azide (Sigma-Aldrich, Steinheim, Germany); purified water (System Elix 3, Millipore, Bedford, USA); carbon tetrachloride (CCl4; J.T Baker, Deventer, Holland); 1,4-dinitrobenzene (DNB); Sigma-Aldrich, Steinheim, Germany); in flame-ionization detection; ultra-high purity hydrogen, ultra-high purity nitrogen; and synthetic air (TEMIS) were used [7].
–O(CH2CH2O)8CHCH2OH; CAS Number 9002-93-1; HLB = 13) (Sigma-Aldrich, Steinheim, Germany); sodium azide (Sigma-Aldrich, Steinheim, Germany); purified water (System Elix 3, Millipore, Bedford, USA); carbon tetrachloride (CCl4; J.T Baker, Deventer, Holland); 1,4-dinitrobenzene (DNB); Sigma-Aldrich, Steinheim, Germany); in flame-ionization detection; ultra-high purity hydrogen, ultra-high purity nitrogen; and synthetic air (TEMIS) were used [7].2.2. Tested Cyclic Siloxanes
2.3. Ex Vivo Human Skin as a Biological Sample
2.4. Apparatus and Chromatographic Conditions
2.5. Preparation of Calibration Standards
2.6. Preparation of Ex Vivo Human Skin and Ex Vivo Penetration and Permeation Studies
2.7. GC-FID
3. Validation of the Analytical Procedure and Acceptance Criteria
3.1. Qualitative Studies
3.2. Quantitative Analysis
3.2.1. Linearity
3.2.2. Precision/Repeatability
3.2.3. Accuracy/Trueness
3.2.4. QC Samples and Recovery (Extraction Efficiency)
3.2.5. LOD and LOQ
3.2.6. Matrix Effect and Matrix Factor
4. Results and Discussion
4.1. Optimization of the Method and Extraction of the Dermal Layers
4.2. Validation of the Analytical Procedure and Acceptance Criteria
4.2.1. Specificity
4.2.2. Precision and Repeatability of the Retention Times
4.2.3. Linearity
4.2.4. Precision/Repeatability
4.2.5. Accuracy/Trueness
4.2.6. Sensitivity of the Assay, LOD, and LOQ
4.2.7. Matrix Effect and Matrix Factor
4.2.8. Quality Control and Recovery (Extraction Efficiency)
4.3. Application of the Validated Method to Real Samples
- (a)
- D5 had a greater affinity for stratum corneum and epidermis, compared to D6.
- (b)
- The greater concentration gradient, compared to D4, was the driving force for the diffusion of D5 from stratum corneum to epidermis. The epidermis-to-stratum corneum ratios were—D4: E/SC = 25, D5: E/SC = 37, and D6: E/SC = 16.
- (c)
- The diffusion of D4 and D5 from epidermis to dermis, and subsequently from dermis to the receptor fluid, was similar, which may indicate their similar affinity for the hydrophilic epidermis and dermis layers, as well as solubility in the receptor fluid. The calculated ratios for D4 were D/SC = 19 and RF/SC = 15, and that for D5 were D/SC = 22 and RF/SC = 12. Definite differences were observed for D6, for which the ratios were calculated as D/SC = 8 and RF/SC = 1. This indicates a significantly diminished diffusion process, despite the fact that the study was conducted in sink conditions.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GC-FID | gas chromatography-flame ionization detector |
| SC | stratum corneum |
| TS | tape-stripping |
| FDA | United States Food and Drug Administration |
| EMA | European Medical Association |
| ICH | International Conference on Harmonization |
| CV | coefficient of variation |
| IS | internal standard |
| MF | matrix factor |
| QCs | quality controls |
| LOQ | limit of quantification |
| LOD | limit of detection |
| LLOQ | lower limit of quantification |
| ULOQ | upper limit of quantification |
| DNB | 1,4-dinitrobenzene |
| D4 | octamethylcyclotetrasiloxane |
| D5 | decamethylcyclopentasiloxane |
| D6 | dodecamethylcyclohexasiloxane |
| LQC | low quality control |
| MQC | medium quality control |
| HQC | high quality control |
| NC | nominal concentration |
| CF | concentration found |
| CD | cumulative dose |
| CE | concentration in the extract |
References
- Bridges, J.; Solomon, K.R. Quantitative weight-of-evidence analysis of the persistence, bioaccumulation, toxicity, and potential for long-range transport of the cyclic volatile methyl siloxanes. J. Toxicol. Environ. Heal. Part B 2016, 19, 345–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojsiewicz-Pieńkowska, K.; Jamrógiewicz, M.; Szymkowska, K.; Krenczkowska, D. Direct Human Contact with Siloxanes (Silicones)-Safety or Risk Part 1. Characteristics of Siloxanes (Silicones). Front. Pharmacol. 2016, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Kurunthachalam, K. Main Uses and Environmental Emissions of Volatile Methylsiloxanes. In Volatile Methylsiloxanes in the Environment; Homem, V., Ratola, N., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Fromme, H. Cyclic Volatile Methylsiloxanes: Occurrence and Exposure. Encycl. Environ. Health 2019, 805–812. [Google Scholar] [CrossRef]
- Rousselle, C. Scientific Committee on Consumer Safety (SCCS) Opinion on Decamethylcyclopentasiloxane (Cyclopentasiloxane, D5) in Cosmetic Products; European Commission Health and Food Safety: Luxemburg, 2016; ISBN 9789279656606. [Google Scholar] [CrossRef]
- Kis, N.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Csányi, E.; Csóka, I.; Berkó, S. Investigation of silicone-containing semisolid in situ film-forming systems using QbD tools. Pharmaceutics 2019, 11, 660. [Google Scholar] [CrossRef] [Green Version]
- Krenczkowska, D.; Mojsiewicz-Pieńkowska, K.; Wielgomas, B.; Cal, K.; Bartoszewski, R.; Bartoszewska, S.; Jankowski, Z. The consequences of overcoming the human skin barrier by siloxanes (silicones) Part 1. Penetration and permeation depth study of cyclic methyl siloxanes. Chemosphere 2019, 231, 607–623. [Google Scholar] [CrossRef]
- Mojsiewicz-Pieńkowska, K.; Krenczkowska, D. Evolution of consciousness of exposure to siloxanes—review of publications. Chemosphere 2018, 191. [Google Scholar] [CrossRef]
- Kim, J.; Mackay, D.; Whelan, M.J. Predicted persistence and response times of linear and cyclic volatile methylsiloxanes in global and local environments. Chemosphere 2018, 195, 325–335. [Google Scholar] [CrossRef]
- Horii, Y.; Nojiri, K.; Minomo, K.; Motegi, M.; Kannan, K. Volatile methylsiloxanes in sewage treatment plants in Saitama, Japan: Mass distribution and emissions. Chemosphere 2019, 233, 677–686. [Google Scholar] [CrossRef]
- Wang, D.-G.; de Solla, S.R.; Lebeuf, M.; Bisbicos, T.; Barrett, G.C.; Alaee, M. Determination of linear and cyclic volatile methylsiloxanes in blood of turtles, cormorants, and seals from Canada. Sci. Total Environ. 2017, 574, 1254–1260. [Google Scholar] [CrossRef]
- Horii, Y.; Minomo, K.; Ohtsuka, N.; Motegi, M.; Nojiri, K.; Kannan, K. Distribution characteristics of volatile methylsiloxanes in Tokyo Bay watershed in Japan: Analysis of surface waters by purge and trap method. Sci. Total Environ. 2017, 586, 56–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, M.; Tian, Y.; Zeng, G. Cyclic volatile methylsiloxanes in sediment, soil, and surface water from Dongting Lake, China. J. Soils Sediments 2018, 18, 2063–2071. [Google Scholar] [CrossRef]
- Tran, T.M.; Hoang, A.Q.; Le, S.T.; Minh, T.B.; Kannan, K. A review of contamination status, emission sources, and human exposure to volatile methyl siloxanes (VMSs) in indoor environments. Sci. Total Environ. 2019, 691, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Kannan, K. Occurrence of cyclic and linear siloxanes in indoor air from Albany, New York, USA, and its implications for inhalation exposure. Sci. Total Environ. 2015, 511, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Abualnaja, K.O.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.-B.; et al. A survey of cyclic and linear siloxanes in indoor dust and their implications for human exposures in twelve countries. Environ. Int. 2015, 78, 39–44. [Google Scholar] [CrossRef]
- Kierkegaard, A.; Bignert, A.; McLachlan, M.S. Cyclic volatile methylsiloxanes in fish from the Baltic Sea. Chemosphere 2013, 93, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Krogseth, I.S.; Whelan, M.J.; Christensen, G.N.; Breivik, K.; Evenset, A.; Warner, N.A. Understanding of Cyclic Volatile Methyl Siloxane Fate in a High Latitude Lake Is Constrained by Uncertainty in Organic Carbon-Water Partitioning. Environ. Sci. Technol. 2017, 51, 401–409. [Google Scholar] [CrossRef]
- Kim, J.; Xu, S. Quantitative structure-reactivity relationships of hydroxyl radical rate constants for linear and cyclic volatile methylsiloxanes. Environ. Toxicol. Chem. 2017, 36, 3240–3245. [Google Scholar] [CrossRef]
- Krogseth, I.; Warner, N.; Christensen, G.; Whelan, M.; Breivik, K.; Evenset, A.; Wasbotten, I. Understanding the Fate and Bioaccumulation of Cyclic Volatile Methyl Siloxanes in Arctic Lakes. Organohalogen Compd. 2014, 76, 186–189. [Google Scholar]
- Warner, N.A.; Evenset, A.; Christensen, G.; Gabrielsen, G.W.; Borgå, K.; Leknes, H. Volatile Siloxanes in the European Arctic: Assessment of Sources and Spatial Distribution. Environ. Sci. Technol. 2010, 44, 7705–7710. [Google Scholar] [CrossRef]
- Krogseth, I.S.; Kierkegaard, A.; McLachlan, M.S.; Breivik, K.; Hansen, K.M.; Schlabach, M. Occurrence and seasonality of cyclic volatile methyl siloxanes in Arctic air. Environ. Sci. Technol. 2013, 47, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Mojsiewicz-Pieńkowska, K. Review of Current Pharmaceutical Applications of Polysiloxanes (Silicones). In Handbook of Polymers for Pharmaceutical Technologies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 363–381. [Google Scholar] [CrossRef]
- Gaj, K. Properties, Potential Toxicity, and Transformations of VMSs in the Environment. In The Handbook of Environmental Chemistry; Springer: Berlin, Heidelberg, Germany, 2018; pp. 1–31. [Google Scholar] [CrossRef]
- European Chemicals Agence Registered Substances-ECHA. Available online: https://echa.europa.eu/information-on-chemicals/registered-substances (accessed on 15 September 2019).
- European Parliament and the Council of the European Union Regulation (Ec) 1272/2008 of the European Parliament and of the Council. Off. J. Eur. Union 2008, L 353/1, 1355.
- European Chemicals Agency. Annex XV Restriction Report Proposal for Restriction: Octamethylcyclotetrasiloxane (D4), Decamethylcyclopentasiloxane (D5), Dodecamethylcyclohexasiloxane (D6); European Chemicals Agency: Helsinki, Finland, 2019.
- European Chemical Agency. Committee for Risk Assessment (RAC) Opinion on an Annex XV Dossier Proposing Restrictions on Four Phthalates; European Chemical Agency: Helsinki, Finland, 2012; pp. 1–26.
- European Commission Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards octamethylcyclotetrasiloxane (D4) and decamethylcyclope. Off. J. Eur. Union 2018, 2018, 45–47.
- Nordic Institute of Product Sustainability Environmental Chemistry and Toxicology Three Siloxanes to be Restricted in Europe. Available online: https://nipsect.dk/three-siloxanes-to-be-restricted-in-europe/ (accessed on 15 September 2019).
- Sanchís, J.; Martínez, E.; Ginebreda, A.; Farré, M.; Barceló, D. Occurrence of linear and cyclic volatile methylsiloxanes in wastewater, surface water and sediments from Catalonia. Sci. Total Environ. 2013, 443, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Capela, D.; Alves, A.; Homem, V.; Santos, L. From the shop to the drain—Volatile methylsiloxanes in cosmetics and personal care products. Environ. Int. 2016, 92–93, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Dudzina, T.; von Goetz, N.; Bogdal, C.; Biesterbos, J.W.H.; Hungerbühler, K. Concentrations of cyclic volatile methylsiloxanes in European cosmetics and personal care products: Prerequisite for human and environmental exposure assessment. Environ. Int. 2014, 62, 86–94. [Google Scholar] [CrossRef]
- Wang, R.; Moody, R.P.; Koniecki, D.; Zhu, J. Low molecular weight cyclic volatile methylsiloxanes in cosmetic products sold in Canada: Implication for dermal exposure. Environ. Int. 2009, 35, 900–904. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, T.; Wang, W.; Kannan, K. Concentrations and assessment of exposure to siloxanes and synthetic musks in personal care products from China. Environ. Pollut. 2011, 159, 3522–3528. [Google Scholar] [CrossRef]
- Hanssen, L.; Warner, N.A.; Braathen, T.; Odland, J.; Lund, E.; Nieboer, E.; Sandanger, T.M. Plasma concentrations of cyclic volatile methylsiloxanes (cVMS) in pregnant and postmenopausal Norwegian women and self-reported use of personal care products (PCPs). Environ. Int. 2013, 51, 82–87. [Google Scholar] [CrossRef]
- Fromme, H.; Cequier, E.; Kim, J.-T.; Hanssen, L.; Hilger, B.; Thomsen, C.; Chang, Y.-S.; Völkel, W. Persistent and emerging pollutants in the blood of German adults: Occurrence of dechloranes, polychlorinated naphthalenes, and siloxanes. Environ. Int. 2015, 85, 292–298. [Google Scholar] [CrossRef]
- Zareba, G.; Gelein, R.; Morrow, P.E.; Utell, M.J. Percutaneous Absorption Studies of Octamethylcyclotetrasiloxane Using the Human Skin/Nude Mouse Model. Skin Pharmacol. Physiol. 2002, 15, 184–194. [Google Scholar] [CrossRef]
- Jovanovic, M.L.; McMahon, J.M.; McNett, D.A.; Tobin, J.M.; Plotzke, K.P. In vitro and In vivo percutaneous absorption of 14C-octamethylcyclotetrasiloxane (14C-D4) and 14C-decamethylcyclopentasiloxane (14C-D5). Regul. Toxicol. Pharmacol. 2008, 50, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Arkles, B. Silicone Fluids: Stable, Inert Media. In Silicon Compounds: Silanes & Silicones; Arkles, B., Larson, G.L., Eds.; Gelest Inc.: Morrisville, PA, USA, 2013; pp. 1–60. [Google Scholar]
- Gobas, F.A.P.C.; Powell, D.E.; Woodburn, K.B.; Springer, T.; Huggett, D.B. Bioaccumulation of decamethylpentacyclosiloxane (D5): A review. Environ. Toxicol. Chem. 2015, 34, 2703–2714. [Google Scholar] [CrossRef]
- Xu, S.; Kozerski, G.; Mackay, D. Critical Review and Interpretation of Environmental Data for Volatile Methylsiloxanes: Partition Properties. Environ. Sci. Technol. 2014, 48, 11748–11759. [Google Scholar] [CrossRef] [PubMed]
- Guth, K.; Schäfer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol. Vitr. 2015, 29, 113–123. [Google Scholar] [CrossRef] [Green Version]
- OECD. OECD Guideline for Thesting of Chemicals. Skin Absorption: In Vitro Method; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies, No 28; OECD: Paris, France, 2004. [Google Scholar]
- World Human Organisation. WHO Environmental Heath Criteria 235 Dermal Absorption; WHO Press: Geneva, Switzerland, 2009; ISBN 978 92 4 157235 4. [Google Scholar]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol. Vitr. 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Davies, D.J.; Heylings, J.R.; McCarthy, T.J.; Correa, C.M. Development of an in vitro model for studying the penetration of chemicals through compromised skin. Toxicol. Vitr. 2015, 29, 176–181. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. Guidance for Industry Bioanalytical Method Validation; Biopharmaceutical Fed Regist; US FDA: Silver Spring, MD, USA, 2001; p. 66.
- European Medicines Agency. Validation of Analytical Procedures: Text and Methodology. Prescrire Int. 2011, 20, 278. [Google Scholar]
- European Medicines Agency. Guideline on bioanalytical method validation. Bioanalysis 2012, 4, 865–868. [Google Scholar]
- Food and Drug Administration. Bioanalytical Method Validation Guidance. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1043, 25. [Google Scholar]
- Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation; Food and Drug Administration; U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM): Rockville, MD, USA, 2001.
- Food and Drug Administration. Guidance for Industry, Bioanalytical Method Validation; Food and Drug Administration, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM): Rockville, MD, USA, 2018; ISBN 3017961508.
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Validation of Analytical Procedures: Text and Methodology ICH Harmonised Tripartite Guideline; ICH: Geneva, Switzerland, 2005. [Google Scholar]
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Guideline M10 on Bioanalytical Method Validation; ICH: Amsterdam, The Netherlands, 2019; Volume 44. [Google Scholar]
- Pereira, M.N.; Matos, B.N.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Development and validation of a simple chromatographic method for simultaneous determination of clindamycin phosphate and rifampicin in skin permeation studies. J. Pharm. Biomed. Anal. 2018, 159, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Kannan, K. Survey of organosilicone compounds, including cyclic and linear siloxanes, in personal-care and household products. Arch. Environ. Contam. Toxicol. 2008, 55, 701–710. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Studies | Validation Parameter | Acceptance Criterion |
|---|---|---|
| Qualitative | Specificity | Blank samples of the appropriate biological matrix should be obtained from at least six individual sources. The response of the interfering compound should be less than 20% of the lowest concentration of the standard curve lower limit of quantification (LLOQ) and 5% standard response internal standard (IS). Blank and zero calibrators should be free of interference at the retention times of the analyte(s) and the IS. Spiked samples should be ± 20% LLOQ. The IS response in the blank should not exceed 5% of the average IS responses of the calibrators and quality controls (QCs). |
| Precision/repeatability/intermediate precision for retention time | Lack of the acceptance criterion in guidelines. Adopted ≤ 2.5%. | |
| Quantitative | Range | Analogically to linearity |
| Linearity | The lowest concentration of analyte from the calibration curve LLOQ ≥ 5 times higher than the blank sample, with a precision of up to 20% of the coefficient of variation (CV) and accuracy within ± 20% (80–120%); upper limit, the highest concentration of the standard curve upper limit of quantification (ULOQ) with a precision below 15% CV and accuracy within ± 15% (85–115%); the correlation coefficient (r) should be greater than 0.99 and the coefficient of determination be (r2) 0.98. Nonzero calibrators should be ± 15% of the nominal (theoretical) concentrations (NC), except at LLOQ where the calibrator should be ± 20% of the nominal concentrations in each validation run. In addition, 75% and a minimum of six nonzero calibrator levels should meet the above criteria in each validation run. | |
| Sensitivity | The lowest nonzero standard on the calibration curve defines the sensitivity (LLOQ). The analyte response at the LLOQ should be ≥ 5 times the analyte response of the zero calibrator. The accuracy should be ± 20% of the nominal concentration (from ≥ 5 replicates in at least 3 runs). The precision should be ± 20% of the CV (from ≥ 5 replicates in at least 3 runs). | |
| LOQ | A 10:1 signal-to-noise ratio. The quantitation limit is generally determined by the analysis of samples with known concentrations of analyte and by establishing the minimum level at which the analyte can be quantified with an acceptable accuracy and precision. | |
| LOD | A 3:1 signal-to-noise ratio. | |
| Precision/Repeatability | Precision should be established with at least 3 independent runs, 4 QC levels per run (LLOQ, L, M, and H QC), and ≥ 5 replicates per QC level. CV in the biological matrix should not exceed 15% and close to 20% of the LLOQ value. | |
| Accuracy/Trueness | Accuracy should be established with at least 3 independent runs, 4 QC levels per run (LLOQ, L, M, and H QC), and ≥ 5 replicates per QC level. The relative error should not exceed ± 15% of the actual value, except for the lowest concentration of the LLOQ analyte close to ± 20% of the value. | |
| QCs | Four QCs, including LLOQ, low (L: defined as three times the LLOQ), mid (M: defined as mid-range), and high (H: defined as high-range) from at least 5 replicates in at least 3 runs. The QCs should be prepared from separate stock solutions than the calibration standards in order to avoid biased estimations that are not related to the analytical performance of the method. | |
| Recovery (extraction efficiency) | Extracted samples at low quality control (LOQ), medium quality control (MOQ), high quality control (HQC) concentrations versus extracts of blanks spiked with the analyte postextraction (maximum of 3 times the LLOQ and close to the HLOQ). Analyte recovery does not have to be 100%, but the analyte and IS recovery rates should be precise and reproducible. The extraction efficiency of an analytical process, reported as a percentage of the known amount of an analyte carried through the sample extraction and processing steps of the method (sample pre-extraction versus sample postextraction). | |
| Matrix effect | Extracted samples including matrix at L, M, and H QC concentrations versus spiked solvent at L, M, and H QC concentrations used for the extraction. It should be assessed by analyzing at least 6 lots of matrix, spiked at a low and at a high level of concentration (maximum of 3 times the LLOQ and close to the ULOQ). The calculated matrix factor (MF) should be 1. The overall CV calculated for the concentration should not be greater than 15%. |
| Cyclic Siloxane | Nominal Concertation NC [µg/mL] | Concentration Found CF (Mean) [µg/mL] | Intra-Day Precision CV [%] n = 6 | Inter-Day Precision CV [%] n = 18 | Accuracy [%] n = 6 | |
|---|---|---|---|---|---|---|
| D4 | LQC | 1 | 1.0 | 2.4 | 3.6 | 102.8 |
| MQC | 5 | 5.0 | 1.5 | 2.2 | 100.4 | |
| HQC | 25 | 24.8 | 0.6 | 1.1 | 99.3 | |
| D5 | LQC | 1 | 1.0 | 6.6 | 4.7 | 106.1 |
| MQC | 5 | 5.1 | 1.7 | 1.9 | 100.44 | |
| HQC | 25 | 25.3 | 0.4 | 1.1 | 100.7 | |
| D6 | LQC | 1 | 1.0 | 2.0 | 6.2 | 105.7 |
| MQC | 5 | 5.1 | 3.5 | 2.9 | 105.6 | |
| HQC | 25 | 24.2 | 6.5 | 5.7 | 99.7 | |
| Siloxane | Heading | Matrix Effect | |||
|---|---|---|---|---|---|
| Nominal Concentration [µg/mL] | Stratum Corneum | Epidermis | Dermis | Receptor Fluid | |
| D4 | 1 | 1.03 | 1.01 | 0.99 | 0.93 |
| 5 | 0.92 | 1.07 | 0.95 | 1.03 | |
| 25 | 0.98 | 1.03 | 1.10 | 1.04 | |
| D5 | 1 | 0.98 | 0.93 | 0.97 | 0.93 |
| 5 | 0.94 | 0.98 | 0.93 | 0.98 | |
| 25 | 0.94 | 0.97 | 0.99 | 0.99 | |
| D6 | 1 | 0.96 | 0.91 | 0.92 | 0.97 |
| 5 | 0.89 | 0.91 | 0.94 | 0.94 | |
| 25 | 0.94 | 0.96 | 0.97 | 1.07 | |
| Cyclic Siloxane | Heading | Sample | Stratum Corneum | Epidermis | Dermis | Receptor Fluid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC [µg/mL] | CF [µg/mL] | CV | Accuracy | CF [µg/mL] | CV | Accuracy | CF [µg/mL] | CV | Accuracy | CF [µg/mL] | CV | Accuracy | ||
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |||||||
| D4 | LQC | 1 | 1.1 | 6.7 | 105.5 | 1.0 | 9.0 | 102.6 | 1.0 | 4.6 | 100.8 | 0.9 | 2.8 | 95.3 |
| MQC | 5 | 4.8 | 4.6 | 95.2 | 5.0 | 9.3 | 100.1 | 4.8 | 4.3 | 95.8 | 5.2 | 4.6 | 103.3 | |
| HQC | 25 | 24.3 | 5.3 | 97.1 | 25.4 | 9.9 | 101.6 | 25.5 | 5.2 | 102.4 | 25.6 | 3.5 | 102.2 | |
| D5 | LQC | 1 | 1.0 | 6.8 | 104.5 | 1.0 | 5.7 | 99.9 | 1.0 | 5.8 | 103.4 | 0.9 | 3.3 | 99.5 |
| MQC | 5 | 4.7 | 7.3 | 94.7 | 5,0 | 10.6 | 99.2 | 4.7 | 5.5 | 93.9 | 4.9 | 5.3 | 98.7 | |
| HQC | 25 | 23.7 | 5.9 | 94.9 | 24.7 | 11.3 | 98.6 | 25.1 | 6.3 | 100.5 | 25.3 | 9.3 | 101.0 | |
| D6 | LQC | 1 | 1.0 | 5.1 | 101.6 | 1,0 | 5.8 | 97.2 | 1.0 | 5.7 | 98.1 | 1.0 | 4.9 | 103.0 |
| MQC | 5 | 4.6 | 9.6 | 92.2 | 4.7 | 7.4 | 94.2 | 4.9 | 6.6 | 97.4 | 4.9 | 7.9 | 97.6 | |
| HQC | 25 | 24.1 | 7.4 | 96.2 | 25.6 | 9.2 | 97.4 | 24.7 | 5.9 | 98.8 | 25.7 | 6.5 | 102.7 | |
| Sample | D4 | D5 | D6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD [µg/cm2/24 h] | CE [µg/mL] | CV [%] | Ratio [%] | CD [µg/cm2/24 h] | CE [µg/mL] | CV [%] | Ratio [%] | CD [µg/cm2/24 h] | CE [µg/mL] | CV [%] | Ratio [%] | |
| Stratum corneum | 27.5 | 3.5 | 4.9 | 25 | 63.9 | 8.1 | 5.5 | 47 | 67.2 | 8.6 | 20.3 | 16 |
| Epidermis | 6.9 | 4.4 | 21.4 | 29.9 | 19.1 | 9.4 | 10.7 | 6.8 | 6.2 | |||
| 74 | 46 | 50 | ||||||||||
| Dermis | 5.1 | 3.2 | 24.5 | 13.9 | 8.8 | 13.5 | 5.3 | 3.4 | 7.4 | |||
| 80 | 53 | 17 | ||||||||||
| Receptor fluid | 4.1 | 2.6 | 27.4 | 7.4 | 4.7 | 24.2 | 0.9 | 0.6 | 29.5 | |||
| TOTAL | 43.6 | 13.7 | 8.8 | 114.1 | 40.7 | 2.6 | 84.1 | 19.4 | 16.3 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krenczkowska, D.; Mojsiewicz-Pieńkowska, K.; Wielgomas, B.; Bazar, D.; Jankowski, Z. Ex Vivo Human Skin is not a Barrier for Cyclic Siloxanes (Cyclic Silicones): Evidence of Diffusion, Bioaccumulation, and Risk of Dermal Absorption Using a New Validated GC-FID Procedure. Pharmaceutics 2020, 12, 586. https://doi.org/10.3390/pharmaceutics12060586
Krenczkowska D, Mojsiewicz-Pieńkowska K, Wielgomas B, Bazar D, Jankowski Z. Ex Vivo Human Skin is not a Barrier for Cyclic Siloxanes (Cyclic Silicones): Evidence of Diffusion, Bioaccumulation, and Risk of Dermal Absorption Using a New Validated GC-FID Procedure. Pharmaceutics. 2020; 12(6):586. https://doi.org/10.3390/pharmaceutics12060586
Chicago/Turabian StyleKrenczkowska, Dominika, Krystyna Mojsiewicz-Pieńkowska, Bartosz Wielgomas, Dagmara Bazar, and Zbigniew Jankowski. 2020. "Ex Vivo Human Skin is not a Barrier for Cyclic Siloxanes (Cyclic Silicones): Evidence of Diffusion, Bioaccumulation, and Risk of Dermal Absorption Using a New Validated GC-FID Procedure" Pharmaceutics 12, no. 6: 586. https://doi.org/10.3390/pharmaceutics12060586