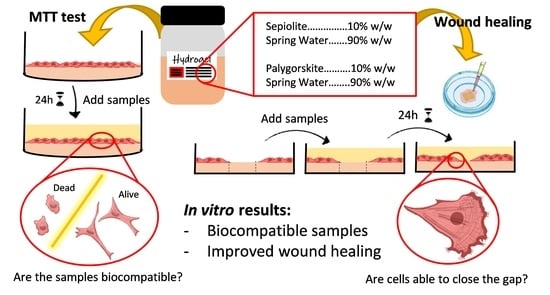
Wound Healing Activity of Nanoclay/Spring Water Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Inorganic Ingredients
2.2.1. Particle Size
2.2.2. Cation Exchange Capacity
2.2.3. Zeta Potential
2.3. In Vitro Tests of Inorganic Ingredients, Spring Waters and Hydrogels
2.3.1. Biocompatibility Tests
2.3.2. Cell Motility Assay for Wound Healing
2.4. Confocal Laser Scanning Microscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Caracterization of Inorganic Ingredients
3.1.1. Particle Size
3.1.2. Cation Exchange Capacity
3.1.3. Zeta Potential
3.2. In Vitro Tests of Inorganic Ingredients, Spring Waters and Hydrogels
3.2.1. Biocompatibility Tests
3.2.2. Cell Motility Assay for Wound Healing
3.3. Confocal Laser Scanning Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olsson, M.; Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Chávez, S.; Nava-Arzaluz, M.G.; Quiroz-Segoviano, R.I.Y.; Ganem-Rondero, A. Nanocarrier-based systems for wound healing. Drug Dev. Ind. Pharm. 2019, 45, 1389–1402. [Google Scholar] [CrossRef]
- García-Villén, F.; Souza, I.M.S.; de Melo Barbosa, R.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; Ojeda-Riascos, S.; Viseras, C. Natural inorganic ingredients in wound healing. Curr. Pharm. Des. 2020, 26, 621–641. [Google Scholar] [CrossRef]
- Tenci, M.; Rossi, S.; Aguzzi, C.; Carazo, E.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Viseras, C.; Caramella, C.M.; Ferrari, F. Carvacrol/clay hybrids loaded into in situ gelling films. Int. J. Pharm. 2017, 531, 676–688. [Google Scholar] [CrossRef]
- García-Villén, F.; Faccendini, A.; Aguzzi, C.; Cerezo, P.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Ruggeri, M.; Ferrari, F.; Sandri, G.; et al. Montmorillonite-norfloxacin nanocomposite intended for healing of infected wounds. Int. J. Nanomed. 2019, 14, 5051–5060. [Google Scholar] [CrossRef] [Green Version]
- Mefteh, S.; Khiari, I.; Sánchez-Espejo, R.; Aguzzi, C.; López-Galindo, A.; Jamoussi, F.; Viseras, C. Characterisation of Tunisian layered clay materials to be used in semisolid health care products. Mater. Technol. 2014, 29, B88–B95. [Google Scholar] [CrossRef]
- García-Villén, F.; Sánchez-Espejo, R.; Carazo, E.; Borrego-Sánchez, A.; Aguzzi, C.; Cerezo, P.; Viseras, C. Characterisation of Andalusian peats for skin health care formulations. Appl. Clay Sci. 2018, 160, 201–205. [Google Scholar] [CrossRef]
- Khiari, I.; Sánchez-Espejo, R.; García-Villén, F.; Cerezo, P.; Aguzzi, C.; López-Galindo, A.; Jamoussi, F.; Viseras, C. Rheology and cation release of tunisian medina mud-packs intended for topical applications. Appl. Clay Sci. 2019, 171, 110–117. [Google Scholar] [CrossRef]
- Rebelo, M.; Viseras, C.; López-Galindo, A.; Rocha, F.; da Silva, E.F. Rheological and thermal characterization of peloids made of selected Portuguese geological materials. Appl. Clay Sci. 2011, 52, 219–227. [Google Scholar] [CrossRef]
- Rebelo, M.; Viseras, C.; López-Galindo, A.; Rocha, F.; da Silva, E.F. Characterization of Portuguese geological materials to be used in medical hydrology. Appl. Clay Sci. 2011, 51, 258–266. [Google Scholar] [CrossRef]
- Sánchez-Espejo, R.; Aguzzi, C.; Cerezo, P.; Salcedo, I.; López-Galindo, A.; Viseras, C. Folk pharmaceutical formulations in western Mediterranean: Identification and safety of clays used in pelotherapy. J. Ethnopharmacol. 2014, 155, 810–814. [Google Scholar] [CrossRef]
- Viseras, C.; Cerezo, P. Aplicación de peloides y fangos termales. In Técnicas y Tecnologías en Hidrología Médica e Hidroterapia; Hernández-Torres, A., Alcázar-Alcázar, R., Eds.; Agencia de Evaluación de Tecnologías Sanitarias - Instituto de Salud Carlos III - Ministerio de Sanidad y Consumo: Madrid, Spain, 2006; pp. 141–146. ISBN 84-95463-33-4. [Google Scholar]
- Elkayam, O.; Ophir, J.; Brener, S.; Paran, D.; Wigler, I.; Efron, D.; Even-Paz, Z.; Politi, Y.; Yaron, M. Immediate and delayed effects of treatment at the Dead Sea in patients with psoriatic arthritis. Rheumatol. Int. 2000, 19, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Delfino, M.; Russo, N.; Migliaccio, G.; Carraturo, N. Experimental study on efficacy of thermal muds of Ischia Island combined with balneotherapy in the treatment of psoriasis vulgaris with plaques. Clin. Ter. 2003, 154, 167–171. [Google Scholar] [PubMed]
- Cozzi, F.; Raffeiner, B.; Beltrame, V.; Ciprian, L.; Coran, A.; Botsios, C.; Perissinotto, E.; Grisan, E.; Ramonda, R.; Oliviero, F.; et al. Effects of mud-bath therapy in psoriatic arthritis patients treated with TNF inhibitors. Clinical evaluation and assessment of synovial inflammation by contrast-enhanced ultrasound (CEUS). Jt. Bone Spine 2015, 82, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Harari, M. Beauty is not only skin deep: The Dead Sea features and cosmetics. An. Hidrol. Médica 2012, 5, 75–88. [Google Scholar]
- Huang, A.; Seité, S.; Adar, T. The use of balneotherapy in dermatology. Clin. Dermatol. 2018, 36, 363–368. [Google Scholar] [CrossRef]
- Riyaz, N.; Arakkal, F. Spa therapy in dermatology. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 128. [Google Scholar] [CrossRef]
- Comacchi, C.; Hercogova, J. A single mud treatment induces normalization of stratum corneum hydration, transepidermal water loss, skin surface pH and sebum content in patients with seborrhoeic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 372–374. [Google Scholar] [CrossRef]
- Williams, L.B.; Haydel, S.E.; Giese, R.F., Jr.; Eberl, D.D. Chemical and Mineralogical Characteristics of French Green Clays Used for Healing. Clays Clay Miner. 2008, 56, 437–452. [Google Scholar] [CrossRef] [PubMed]
- López-Galindo, A.; Viseras, C.; Aguzzi, C.; Cerezo, P. Pharmaceutical and cosmetic uses of fibrous clays. In Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 299–324. ISBN 9780444536075. [Google Scholar]
- Argenziano, G.; Delfino, M.; Russo, N. Mud and baththerapy in the acne cure. Clin. Ter. 2004, 155, 125. [Google Scholar]
- Cézanne, L.; Gaboriau, F.; Charveron, M.; Morlière, P.; Tocanne, J.F.; Dubertret, L. Effects of the Avène spring water on the dynamics of lipids in the membranes of cultured fibroblasts. Skin Pharmacol. 1993, 6, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Mahe, Y.F.; Perez, M.J.; Tacheau, C.; Fanchon, C.; Martin, R.; Rousset, F.; Seite, S. A new Vitreoscilla filiformis extract grown on spa water-enriched medium activates endogenous cutaneous antioxidant and antimicrobial defenses through a potential Toll-like receptor 2/protein kinase C, zeta transduction pathway. Clin. Cosmet. Investig. Dermatol. 2013, 6, 191–196. [Google Scholar]
- Mahé, Y.F.; Martin, R.; Aubert, L.; Billoni, N.; Collin, C.; Pruche, F.; Bastien, P.; Drost, S.S.; Lane, A.T.; Meybeck, A. Induction of the skin endogenous protective mitochondrial MnSOD by Vitreoscilla filiformis extract. Int. J. Cosmet. Sci. 2006, 28, 277–287. [Google Scholar] [CrossRef]
- Castex-Rizzi, N.; Charveron, M.; Merial-Kieny, C. Inhibition of TNF-alpha induced-adhesion molecules by Avène Thermal Spring Water in human endothelial cells. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 6–11. [Google Scholar] [CrossRef]
- Moysan, A.; Morlière, P.; Marquis, I.; Richard, A.; Dubertret, L. Effects of selenium on UVA-Induced lipid peroxidation in cultured human skin fibroblasts. Skin Pharmacol. Physiol. 1995, 8, 139–148. [Google Scholar] [CrossRef]
- Chebassier, N.; Ouijja, E.H.; Viegas, I.; Dreno, B. Stimulatory effect of boron and manganese salts on keratinocyte migration. Acta Derm. Venereol. 2004, 84, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Kang, D.; Wang, Y.; Yu, Y.; Fan, J.; Takashi, E. Carbonate ion-enriched hot spring water promotes skin wound healing in nude rats. PLoS ONE 2015, 10, e0117106. [Google Scholar] [CrossRef] [Green Version]
- Chiarini, A.; Dal Pra, I.; Pacchiana, R.; Zumiani, G.; Zanoni, M.; Armato, U. Comano’s (Trentino) thermal water interferes with interleukin-6 production and secretion and with cytokeratin-16 expression by cultured human psoriatic keratinocytes: Further potential mechanisms of its anti-psoriatic action. Int. J. Mol. Med. 2006, 18, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Faga, A.; Nicoletti, G.; Gregotti, C.; Finotti, V.; Nitto, A.; Gioglio, L. Effects of thermal water on skin regeneration. Int. J. Mol. Med. 2012, 29, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Saler, M.; Pellegatta, T.; Tresoldi, M.M.; Bonfanti, V.; Malovini, A.; Faga, A.; Riva, F. Ex vivo regenerative effects of a spring water. Biomed. Rep. 2017, 7, 508–514. [Google Scholar] [PubMed] [Green Version]
- Sánchez-Espejo, R.; Aguzzi, C.; Salcedo, I.; Cerezo, P.; Viseras, C. Clays in complementary and alternative medicine. Mater. Technol. 2014, 29, B78–B81. [Google Scholar] [CrossRef]
- Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C.; López-Galindo, A.; Machado, J.; Viseras, C. Physicochemical and in vitro cation release relevance of therapeutic muds “maturation”. Appl. Clay Sci. 2015, 116–117, 1–7. [Google Scholar] [CrossRef]
- Khiari, I.; Mefteh, S.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C.; López-Galindo, A.; Jamoussi, F.; Viseras Iborra, C. Study of traditional Tunisian medina clays used in therapeutic and cosmetic mud-packs. Appl. Clay Sci. 2014, 101, 141–148. [Google Scholar] [CrossRef]
- Lizarbe, M.A.; Olmo, N.; Gavilanes, J.G. Outgrowth of fibroblasts on sepiolite-collagen complex. Biomaterials 1987, 8, 35–37. [Google Scholar] [CrossRef]
- Olmo, N.; Lizarbe, M.A.; Gavilanes, J.G. Biocompatibility and degradability of sepiolite-collagen complex. Biomaterials 1987, 8, 67–69. [Google Scholar] [CrossRef]
- Kommireddy, D.S.; Ichinose, I.; Lvov, Y.M.; Mills, D.K. Nanoparticle Multilayers: Surface Modification for Cell Attachment and Growth. J. Biomed. Nanotechnol. 2006, 1, 286–290. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA-clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O.C. Clay: New opportunities for tissue regeneration and biomaterial design. Adv. Mater. 2013, 25, 4069–4086. [Google Scholar] [CrossRef]
- Aguzzi, C.; Sandri, G.; Cerezo, P.; Carazo, E.; Viseras, C. Health and medical applications of tubular clay minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 7, pp. 708–725. ISBN 9780081002933. [Google Scholar]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Aguzzi, C.; Viseras, C.; Caramella, C. Clay minerals for tissue regeneration, repair, and engineering. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 385–402. ISBN 9781782424567. [Google Scholar]
- Ishikawa, K.; Akasaka, T.; Abe, S.; Yawaka, Y.; Suzuki, M.; Watari, F. Application of Imogolite, Almino-Silicate Nanotube, as Scaffold for the Mineralization of Osteoblasts. Bioceram. Dev. Appl. 2010, 1, 1–3. [Google Scholar] [CrossRef]
- Ishikawa, K.; Akasaka, T.; Yawaka, Y.; Watari, F. High functional expression of osteoblasts on imogolite, aluminosilicate nanotubes. J. Biomed. Nanotechnol. 2010, 6, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Saravana, P.S.; Chun, B.S.; Kang, H.W. Fabrication of multifunctional chitosan-based nanocomposite film with rapid healing and antibacterial effect for wound management. Int. J. Biol. Macromol. 2018, 118, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, I.; Aguzzi, C.; Sandri, G.; Bonferoni, M.C.; Mori, M.; Cerezo, P.; Sánchez, R.; Viseras, C.; Caramella, C. In vitro biocompatibility and mucoadhesion of montmorillonite chitosan nanocomposite: A new drug delivery. Appl. Clay Sci. 2012, 55, 131–137. [Google Scholar] [CrossRef]
- Li, P.-R.; Wei, J.-C.; Chiu, Y.-F.; Su, H.-L.; Peng, F.-C.; Lin, J.-J. Evaluation on cytotoxicity and genotoxicity of the exfoliated silicate nanoclay. ACS Appl. Mater. Interfaces 2010, 2, 1608–1613. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Guidi, P.; Bonelli, B.; Bernardeschi, M.; Bianchi, M.G.; Esposito, S.; Frenzilli, G.; Lucchesi, P.; Nigro, M.; Scarcelli, V.; et al. Imogolite: An aluminosilicate nanotube endowed with low cytotoxicity and genotoxicity. Chem. Res. Toxicol. 2014, 27, 1142–1154. [Google Scholar] [CrossRef]
- Lai, X.; Agarwal, M.; Lvov, Y.M.; Pachpande, C.; Varahramyan, K.; Witzmann, F.A. Proteomic profiling of halloysite clay nanotube exposure in intestinal cell co-culture. J. Appl. Toxicol. 2013, 33, 1316–1329. [Google Scholar] [CrossRef] [Green Version]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Aguzzi, C.; Mori, M.; Grisoli, P.; Cerezo, P.; Tenci, M.; Viseras, C.; et al. Montmorillonite-chitosan-silver sulfadiazine nanocomposites for topical treatment of chronic skin lesions: In vitro biocompatibility, antibacterial efficacy and gap closure cell motility properties. Carbohydr. Polym. 2014, 102, 970–977. [Google Scholar] [CrossRef]
- Maisanaba, S.; Pichardo, S.; Puerto, M.; Gutiérrez-Praena, D.; Cameán, A.M.; Jos, A. Toxicological evaluation of clay minerals and derived nanocomposites: A review. Environ. Res. 2015, 138, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Sathi, G.A.; Yamamoto, O. Wound healing effect of bioactive ion released from Mg-smectite. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 77, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Luo, Y.; Wang, S.; Shen, M.; Tomás, H.; Zhu, M.; Shi, X. Attapulgite-doped electrospun poly(lactic-co-glycolic acid) nanofibers enable enhanced osteogenic differentiation of human mesenchymal stem cells. RSC Adv. 2015, 5, 2383–2391. [Google Scholar] [CrossRef]
- Cervini-Silva, J.; Nieto-Camacho, A.; Gómez-Vidales, V. Oxidative stress inhibition and oxidant activity by fibrous clays. Colloids Surf. B Biointerfaces 2015, 133, 32–35. [Google Scholar] [CrossRef]
- Cervini-Silva, J.; Nieto-Camacho, A.; Ramírez-Apan, M.T.; Gómez-Vidales, V.; Palacios, E.; Montoya, A.; Ronquillo de Jesús, E. Anti-inflammatory, anti-bacterial, and cytotoxic activity of fibrous clays. Colloids Surf. B Biointerfaces 2015, 129, 1–6. [Google Scholar] [CrossRef]
- Nasirov, M.I.; Efendieva, F.M.; Ismaĭlova, D.A. The influence of peloids from volcanic deposits in Azerbaijan on the dynamics of sugar content in blood and urine and the wound healing in patients at the early stages of diabetic gangrene of the lower extremities. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2009, 42–43. [Google Scholar]
- Abu-al-Basal, M.A. Histological evaluation of the healing properties of Dead Sea black mud on full-thickness excision cutaneous wounds in BALB/c mice. Pakistan J. Biol. Sci. 2012, 15, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Dário, G.M.I.; Da Silva, G.G.; Gonçalves, D.L.; Silveira, P.; Junior, A.T.; Angioletto, E.; Bernardin, A.M. Evaluation of the healing activity of therapeutic clay in rat skin wounds. Mater. Sci. Eng. C 2014, 43, 109–116. [Google Scholar] [CrossRef]
- García-Villén, F.; Sánchez-Espejo, R.; López-Galindo, A.; Cerezo, P.; Viseras, C. Design and characterization of spring water hydrogels with natural inorganic excipients. Appl. Clay Sci. 2020. Manuscript under review. [Google Scholar]
- Diputación Provincial de Granada; Instituto Tecnológico Geominero de España. Atlas Hidrogeológico de la Provincia de Granada. 1990. Available online: http://aguas.igme.es/igme/publica/libro75/lib_75.htm (accessed on 24 April 2020).
- Maraver Eyzaguirre, F.; Armijo de Castro, F. Vademécum II de Aguas Mineromedicinales Españolas; Maraver Eyzaguirre, F., Armijo Castro, F., Eds.; Editorial Complutense: Madrid, Spain, 2010; ISBN 9788474919981. [Google Scholar]
- Klinkenberg, M.; Rickertsen, N.; Kaufhold, S.; Dohrmann, R.; Siegesmund, S. Abrasivity by bentonite dispersions. Appl. Clay Sci. 2009, 46, 37–42. [Google Scholar] [CrossRef]
- Quintela, A.; Costa, C.; Terroso, D.; Rocha, F. Abrasiveness index of dispersions of Portuguese clays using the Einlehner method: Influence of clay parameters. Clay Miner. 2014, 49, 27–34. [Google Scholar] [CrossRef]
- Ganfoud, R.; Puchot, L.; Fouquet, T.; Verge, P. H-bonding supramolecular interactions driving the dispersion of kaolin into benzoxazine: A tool for the reinforcement of polybenzoxazines thermal and thermo-mechanical properties. Compos. Sci. Technol. 2015, 110, 1–7. [Google Scholar] [CrossRef]
- Santos, S.C.R.; Boaventura, R.A.R. Adsorption of cationic and anionic azo dyes on sepiolite clay: Equilibrium and kinetic studies in batch mode. J. Environ. Chem. Eng. 2016, 4, 1473–1483. [Google Scholar] [CrossRef]
- Álvarez, A.; Santarén, J.; Esteban-Cubillo, A.; Aparicio, P. Current Industrial Applications of Palygorskite and Sepiolite. In Developments in Palygorskite-Sepiolite Research. A New Outlook of These Nanomaterials; Galán, E., Singer, A., Eds.; Elsevier B.V: Oxford, UK, 2011; pp. 281–298. ISBN 978-0-444-53607-5. [Google Scholar]
- McLean, S.A.; Allen, B.L.; Craig, J.R. The Occurrence of Sepiolite and Attapulgite on the Southern High Plains. Clays Clay Miner. 1972, 20, 143–149. [Google Scholar] [CrossRef]
- Galan, E. Properties and applications of palygorskite-sepiolite clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Zeng, H.F.; Lin, L.J.; Xi, Y.M.; Han, Z.Y. Effects of raw and heated palygorskite on rumen fermentation in vitro. Appl. Clay Sci. 2017, 138, 125–130. [Google Scholar] [CrossRef]
- Lobato-Aguilar, H.; Uribe-Calderón, J.A.; Herrera-Kao, W.; Duarte-Aranda, S.; Baas-López, J.M.; Escobar-Morales, B.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Synthesis, characterization and chlorhexidine release from either montmorillonite or palygorskite modified organoclays for antibacterial applications. J. Drug Deliv. Sci. Technol. 2018, 46, 452–460. [Google Scholar] [CrossRef]
- Shariatmadari, H. Sorption of Selected Cationic and Neutral Organic Molecules on Palygorskite and Sepiolite. Clays Clay Miner. 1999, 47, 44–53. [Google Scholar] [CrossRef]
- Rytwo, G.; Nir, S.; Crespin, M.; Margulies, L. Adsorption and Interactions of Methyl Green with Montmorillonite and Sepiolite. J. Colloid Interface Sci. 2000, 222, 12–19. [Google Scholar] [CrossRef]
- Lemić, J.; Tomašević-Čanović, M.; Djuričić, M.; Stanić, T. Surface modification of sepiolite with quaternary amines. J. Colloid Interface Sci. 2005, 292, 11–19. [Google Scholar] [CrossRef]
- Shirvani, M.; Shariatmadari, H.; Kalbasi, M.; Nourbakhsh, F.; Najafi, B. Sorption of cadmium on palygorskite, sepiolite and calcite: Equilibria and organic ligand affected kinetics. Colloids Surf. A Physicochem. Eng. Asp. 2006, 287, 182–190. [Google Scholar] [CrossRef]
- Al-Futaisi, A.; Jamrah, A.; Al-Rawas, A.; Al-Hanai, S. Adsorption capacity and mineralogical and physico-chemical characteristics of Shuwaymiyah palygorskite (Oman). Environ. Geol. 2007, 51, 1317–1327. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Yu, T.-L.; Munkhbayer, S.; Kuo, T.-H.; Hung, Y.-C.; Jean, J.-S.; Lin, K.-H. Sorptive removal of tetracycline from water by palygorskite. J. Hazard. Mater. 2009, 165, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rhouta, B.; Zatile, E.; Bouna, L.; Lakbita, O.; Maury, F.; Daoudi, L.; Lafont, M.C.; Amjoud, M.; Senocq, F.; Jada, A.; et al. Comprehensive physicochemical study of dioctahedral palygorskite-rich clay from Marrakech High Atlas (Morocco). Phys. Chem. Miner. 2013, 40, 411–424. [Google Scholar] [CrossRef] [Green Version]
- Paolisso, G.; Barbagallo, M. Hypertension, diabetes mellitus, and insulin resistance. The role of intracellular magnesium. Am. J. Hypertens. 1997, 10, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Tateo, F.; Ravaglioli, A.; Andreoli, C.; Bonina, F.; Coiro, V.; Degetto, S.; Giaretta, A.; Menconi Orsini, A.; Puglia, C.; Summa, V. The in-vitro percutaneous migration of chemical elements from a thermal mud for healing use. Appl. Clay Sci. 2009, 44, 83–94. [Google Scholar] [CrossRef]
- De Gomes, C.S.F.; Silva, J.B.P. Minerals and clay minerals in medical geology. Appl. Clay Sci. 2007, 36, 4–21. [Google Scholar] [CrossRef]
- Lansdown, A.B.G.; Sampson, B.; Rowe, A. Sequential changes in trace metal, metallothionein and calmodulin concentrations in healing skin wounds. J. Anat. 1999, 195, 375–386. [Google Scholar] [CrossRef]
- Dubé, J.; Rochette-Drouin, O.; Lévesque, P.; Gauvin, R.; Roberge, C.J.; Auger, F.A.; Goulet, D.; Bourdages, M.; Plante, M.; Germain, L.; et al. Restoration of the transepithelial potential within tissue-engineered human skin in vitro and during the wound healing process in vivo. Tissue Eng. Part A 2010, 16, 3055–3063. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Biphasic responses of human vascular smooth muscle cells to magnesium ion. J. Biomed. Mater. Res. Part A 2016, 104, 347–356. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Calcium: A potential central regulator in wound healing in the skin. Wound Repair Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Fairley, J.A.; Marcelo, C.L.; Hogan, V.A.; Voorhees, J.J. Increased calmodulin levels in psoriasis and low Ca++ regulated mouse epidermal keratinocyte cultures. J. Investig. Dermatol. 1985, 84, 195–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karvonen, S.L.; Korkiamäki, T.; Ylä-Outinen, H.; Nissinen, M.; Teerikangas, H.; Pummi, K.; Karvonen, J.; Peltonen, J. Psoriasis and altered calcium metabolism: Downregulated capacitative calcium influx and defective calcium-mediated cell signaling in cultured psoriatic keratinocytes. J. Investig. Dermatol. 2000, 114, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Jin, X. Needle-punched three-dimensional nonwoven wound dressings with density gradient from biocompatible calcium alginate fiber. Text. Res. J. 2019, 89, 2776–2788. [Google Scholar] [CrossRef]
- Hotta, E.; Hara, H.; Kamiya, T.; Adachi, T. Non-thermal atmospheric pressure plasma-induced IL-8 expression is regulated via intracellular K + loss and subsequent ERK activation in human keratinocyte HaCaT cells. Arch. Biochem. Biophys. 2018, 644, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Lim, J.W.; Kim, B.K.; Park, S.J.; Kim, S.W.; Choi, T.H. KCl mediates K+ channel-activated mitogen activated protein kinases signaling in wound healing. Arch. Plast. Surg. 2015, 42, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, M.; Sun, D.; Li, Y.; Wu, T. Effective removal of emulsified oil from oily wastewater using surfactant-modified sepiolite. Appl. Clay Sci. 2018, 157, 227–236. [Google Scholar] [CrossRef]
- Di Credico, B.; Tagliaro, I.; Cobani, E.; Conzatti, L.; D’Arienzo, M.; Giannini, L.; Mascotto, S.; Scotti, R.; Stagnaro, P.; Tadiello, L. A Green Approach for Preparing High-Loaded Sepiolite/Polymer Biocomposites. Nanomaterials 2019, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Middea, A.; Spinelli, L.S.; Souza, F.G.; Neumann, R.; da Gomes, O.F.M.; Fernandes, T.L.A.P.; de Lima, L.C.; Barthem, V.M.T.S.; de Carvalho, F.V. Synthesis and characterization of magnetic palygorskite nanoparticles and their application on methylene blue remotion from water. Appl. Surf. Sci. 2015, 346, 232–239. [Google Scholar] [CrossRef]
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C.M. The relationship between pH and zeta potential of ∼30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Spriano, S.; Sarath Chandra, V.; Cochis, A.; Uberti, F.; Rimondini, L.; Bertone, E.; Vitale, A.; Scolaro, C.; Ferrari, M.; Cirisano, F.; et al. How do wettability, zeta potential and hydroxylation degree affect the biological response of biomaterials? Mater. Sci. Eng. C 2017, 74, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems - A review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Takeuchi, K.I.; Ishihara, M.; Kawaura, C.; Noji, M.; Furuno, T.; Nakanishi, M. Effect of zeta potential of cationic liposomes containing cationic cholesterol derivatives on gene transfection. FEBS Lett. 1996, 397, 207–209. [Google Scholar] [CrossRef] [Green Version]
- Bengali, Z.; Pannier, A.K.; Segura, T.; Anderson, B.C.; Jang, J.-H.; Mustoe, T.A.; Shea, L.D. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol. Bioeng. 2005, 90, 290–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdevices 2008, 10, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Luo, J. Biopolymer/montmorillonite nanocomposite: Preparation, drug-controlled release property and cytotoxicity. Nanotechnology 2008, 19, 065707. [Google Scholar] [CrossRef]
- Sabuncu, A.C.; Grubbs, J.; Qian, S.; Abdel-Fattah, T.M.; Stacey, M.W.; Beskok, A. Probing nanoparticle interactions in cell culture media. Colloids Surf. B Biointerfaces 2012, 95, 96–102. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.; Jesus, S.; Bernardi, N.; Colaço, M.; Borges, O. Poly(D, L-lactic Acid) nanoparticle size reduction increases its immunotoxicity. Front. Bioeng. Biotechnol. 2019, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- de Souza e Silva, J.M.; Hanchuk, T.D.M.; Santos, M.I.; Kobarg, J.; Bajgelman, M.C.; Cardoso, M.B. Viral inhibition mechanism mediated by surface-modified silica nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16564–16572. [Google Scholar] [CrossRef]
- Su, Y.; Liao, J.L.; Wang, F. Effect of Orient House-Chuen, a concentrate of deep underground mineral spring water, on proliferation and tyrosinase activity of melanocytes. Chin. J. Biol. 2010, 23, 964–966. [Google Scholar]
- Fukushima, K.; Rasyida, A.; Yang, M.C. Characterization, degradation and biocompatibility of PBAT based nanocomposites. Appl. Clay Sci. 2013, 80–81, 291–298. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Antunes, F.; Pires, J. Sepiolite based materials for storage and slow release of nitric oxide. New J. Chem. 2013, 37, 4052–4060. [Google Scholar] [CrossRef]
- Toledano-Magaña, Y.; Flores-Santos, L.; Montes de Oca, G.; González-Montiel, A.; Laclette, J.-P.; Carrero, J.-C. Effect of Clinoptilolite and Sepiolite Nanoclays on Human and Parasitic Highly Phagocytic Cells. Biomed Res. Int. 2015, 2015, 164980. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, C.; Sánchez-Espejo, R.; Cerezo, P.; Machado, J.; Bonferoni, C.; Rossi, S.; Salcedo, I.; Viseras, C. Networking and rheology of concentrated clay suspensions “matured” in mineral medicinal water. Int. J. Pharm. 2013, 453, 473–479. [Google Scholar] [CrossRef]
- Staffieri, A.; Marino, F.; Staffieri, C.; Giacomelli, L.; D’Alessandro, E.; Maria Ferraro, S.; Fedrazzoni, U.; Marioni, G. The effects of sulfurous-arsenical-ferruginous thermal water nasal irrigation in wound healing after functional endoscopic sinus surgery for chronic rhinosinusitis: A prospective randomized study. Am. J. Otolaryngol. 2008, 29, 223–229. [Google Scholar] [CrossRef]
- Davinelli, S.; Bassetto, F.; Vitale, M.; Scapagnini, G. Thermal Waters and the Hormetic Effects of Hydrogen Sulfide on Inflammatory Arthritis and Wound Healing; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128142530. [Google Scholar]
- Guzmán, R.; Campos, C.; Yuguero, R.; Masegù, C.; Gil, P.; Moragón, Á.C. Protective effect of sulfurous water in peripheral blood mononuclear cells of Alzheimer’s disease patients. Life Sci. 2015, 132, 61–67. [Google Scholar] [CrossRef]
- Lin, F.H.; Chen, C.H.; Cheng, W.T.K.; Kuo, T.F. Modified montmorillonite as vector for gene delivery. Biomaterials 2006, 27, 3333–3338. [Google Scholar] [CrossRef]
- Abduljauwad, S.N.; Ahmed, H.-R. Enhancing cancer cell adhesion with clay nanoparticles for countering metastasis. Sci. Rep. 2019, 9, 5935. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ramasamy, K.; Lim, S.M.; Ismail, M.F.; Majeed, A.B.A. Antimicrobial and in vitro wound healing properties of novel clay based bionanocomposite films. J. Mater. Sci. Mater. Med. 2014, 25, 1925–1939. [Google Scholar] [CrossRef]
- Vaiana, C.A.; Leonard, M.K.; Drummy, L.F.; Singh, K.M.; Bubulya, A.; Vaia, R.A.; Naik, R.R.; Kadakia, M.P. Epidermal growth factor: Layered silicate nanocomposites for tissue regeneration. Biomacromolecules 2011, 12, 3139–3146. [Google Scholar] [CrossRef]
- de Gois da Silva, M.L.; Fortes, A.C.; Oliveira, M.E.R.; de Freitas, R.M.; da Silva Filho, E.C.; de La Roca Soares, M.F.; Soares-Sobrinho, J.L.; da Silva Leite, C.M. Palygorskite organophilic for dermopharmaceutical application. J. Therm. Anal. Calorim. 2014, 115, 2287–2294. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.K.; Wong, T.W.; Thomas, S.; Grohens, Y. Natural polymer/inorganic material based hybrid scaffolds for skin wound healing. Polym. Rev. 2015, 55, 453–490. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Peng, F.-C.; Chiu, Y.-F.; Lee, H.-C.; Chen, C.-W.; Wei, J.-C.; Lin, J.-J. Nanohybrids of Silver Particles Immobilized on Silicate Platelet for Infected Wound Healing. PLoS ONE 2012, 7, e38360. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in pelotherapy. A review. Part II: Organic compounds, microbiology and medical applications. Appl. Clay Sci. 2020, 189, 105531. [Google Scholar] [CrossRef]
- Sandri, G.; Faccendini, A.; Longo, M.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Prina-Mello, A.; Aguzzi, C.; Viseras, C.; et al. Halloysite-and montmorillonite-loaded scaffolds as enhancers of chronic wound healing. Pharmaceutics 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtimaki, J.; Hakala, M.; Lappalainen, P. Actin filament structures in migrating cells. Handb. Exp. Pharmacol. 2017, 235, 1–30. [Google Scholar]









| PS9 | G30 | |||
|---|---|---|---|---|
| Mineralogical Composition | Sepiolite | 92% | Palygorskite | 58% |
| Muscovite | 8% | Quartz | 26% | |
| Fluorapatite | 7% | |||
| Smectites and sepiolite | 6% | |||
| Calcite/dolomite | 3% | |||
| ALI | GR | ||
|---|---|---|---|
| pH ± s.d. | 7.90 ± 0.0472 | 8.02 ± 0.0823 | |
| Conductivity (µS/cm) ± s.d. | 2251.5 ± 6.74537 | 2465.5 ± 8.89482 | |
| Elemental Composition | Ca (mg/L) | 348.00 | 460.00 |
| Mg (mg/L) | 109.0 | 88.00 | |
| Na (mg/L) | 57.00 | 27.40 | |
| K (mg/L) | 4.60 | 6.80 | |
| B (µg/L) | 25.00 | 12.00 | |
| Ba (µg/L) | 18.80 | 13.00 | |
| Cr (µg/L) | 4.3 | 1 | |
| Zn (µg/L) | 464.99 | 301.08 | |
| Mn (µg/L) | <1 | 108.66 | |
| Li (µg/L) | 244.2 | 65.00 | |
| Ni (µg/L) | 9.4 | 5.20 | |
| Fe (µg/L) | 6.00 | 21.00 | |
| Se (µg/L) | 2.3 | 1.00 |
| Identification Code | Composition |
|---|---|
| PS9ALI | 10% w/w PS9, 90% w/w ALI |
| PS9GR | 10% w/w PS9, 90% w/w GR |
| G30ALI | 10% w/w G30, 90% w/w ALI |
| G30GR | 10% w/w G30, 90% w/w GR |
| PS9 | G30 | |
|---|---|---|
| d10 (µm) | 4.0 ± 0.07 | 4.8 ± 0.03 |
| d50 (µm) | 9.9 ± 0.15 | 20.2 ± 0.03 |
| d90 (µm) | 23.9 ± 0.2 | 49.3 ± 0.10 |
| SPAN Factor | 2.0 ± 0.02 | 2.2 ± 0.01 |
| Main Mode (µm) | 8.9 | 28.3 |
| mEq/100g | PS9 | G30 |
|---|---|---|
| Na+ | 0.70 ± 0.045 | 1.68 ± 0.071 |
| K+ | 0.33 ± 0.023 | 0.13 ± 0.032 |
| Mg2+ | 3.62 ± 0.341 | 7.61± 0.326 |
| Ca2+ | 4.53 ± 0.123 | 6.87± 0.186 |
| Total | 9.18 | 16.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Villén, F.; Faccendini, A.; Miele, D.; Ruggeri, M.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Rossi, S.; Viseras, C.; Sandri, G. Wound Healing Activity of Nanoclay/Spring Water Hydrogels. Pharmaceutics 2020, 12, 467. https://doi.org/10.3390/pharmaceutics12050467
García-Villén F, Faccendini A, Miele D, Ruggeri M, Sánchez-Espejo R, Borrego-Sánchez A, Cerezo P, Rossi S, Viseras C, Sandri G. Wound Healing Activity of Nanoclay/Spring Water Hydrogels. Pharmaceutics. 2020; 12(5):467. https://doi.org/10.3390/pharmaceutics12050467
Chicago/Turabian StyleGarcía-Villén, Fátima, Angela Faccendini, Dalila Miele, Marco Ruggeri, Rita Sánchez-Espejo, Ana Borrego-Sánchez, Pilar Cerezo, Silvia Rossi, César Viseras, and Giuseppina Sandri. 2020. "Wound Healing Activity of Nanoclay/Spring Water Hydrogels" Pharmaceutics 12, no. 5: 467. https://doi.org/10.3390/pharmaceutics12050467