Mepirapim, a Novel Synthetic Cannabinoid, Induces Addiction-Related Behaviors through Neurochemical Maladaptation in the Brain of Rodents
Abstract
:1. Introduction
2. Results
2.1. Mepirapim Treatment Supported Maintenance of IVSA in Rats
2.2. Mepirapim Treatment Produced CPP in Mice
2.3. Mepirapim Treatment Induced CB Tetrad-Related Symptoms in Mice
2.4. Mepirapim Treatment Induced Changes in Extracellular Levels of DA and Its Metabolites (DOPAC and HVA) in Rat NAc
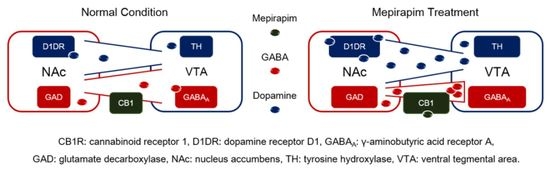
2.5. Mepirapim Treatment Induced Changes in Addiction-Related Molecules in Mice VTA and NAc
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Abuse Potential Assessments
4.3.1. IVSA Test
Apparatus
Food Training
Intravenous Catheterization
IVSA Test for 7 Days
4.3.2. CPP Test
4.4. Assessment of CB Tetrad-Related Symptoms
4.4.1. Open Field Test
4.4.2. Body Temperature Measurement
4.5. Microdialysis
4.5.1. Chemicals
4.5.2. Probe Implantation
4.5.3. Brain Microdialysis
4.5.4. LC-MS/MS Analysis
4.6. Western Blot
4.7. Neurotransmitter Enzyme-Linked Immunosorbent Assay
4.7.1. GABA Enzyme-Linked Immunosorbent Assay
4.7.2. DA ELISA
4.8. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNODC. World Drug Report 2021, 3rd ed.; United Nations Office on Drugs and Crime: Vienna, Austria, 2021; p. 121. [Google Scholar]
- Panlilio, L.V.; Goldberg, S.R.; Justinova, Z. Cannabinoid abuse and addiction: Clinical and preclinical findings. Clin. Pharmacol. Ther. 2015, 97, 616–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, C.H. Pharmacology and effects of cannabis: A brief review. Br. J. Psychiatry 2001, 178, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNODC. Current NPS Threats; United Nations Office on Drugs and Crime Early Warning Advisory: Vienna, Austria, 2021; p. 6. [Google Scholar]
- Tai, S.; Fantegrossi, W.E. Synthetic cannabinoids: Pharmacology, behavioral effects, and abuse potential. Curr. Addict. Rep. 2014, 1, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weinstein, A.M. Synthetic and non-synthetic cannabinoid drugs and their adverse effects-a review from public health prospective. Front. Public Health 2018, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.; DeLollis, M.; Shad, M.U. Potential treatment for overdose with synthetic cannabinoids. Med. Cannabis Cannabinoids 2020, 3, 25–26. [Google Scholar] [CrossRef]
- Harris, C.R.; Brown, A. Synthetic Cannabinoid Intoxication: A Case Series and Review. J. Emerg. Med. 2013, 44, 360–366. [Google Scholar] [CrossRef]
- Macfarlane, V.; Christie, G. Synthetic cannabinoid withdrawal: A new demand on detoxification services. Drug Alcohol Rev. 2015, 34, 147–153. [Google Scholar] [CrossRef]
- Maldonado, R.; Valverde, O.; Berrendero, F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006, 29, 225–232. [Google Scholar] [CrossRef]
- Szabo, B.; Siemes, S.; Wallmichrath, I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur. J. Neurosci. 2002, 15, 2057–2061. [Google Scholar] [CrossRef]
- Canazza, I.; Ossato, A.; Trapella, C.; Fantinati, A.; De Luca, M.A.; Margiani, G.; Vincenzi, F.; Rimondo, C.; Di Rosa, F.; Gregori, A.; et al. Effect of the novel synthetic cannabinoids AKB48 and 5F-AKB48 on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. In vitro and in vivo pharmacological studies. Psychopharmacology 2016, 233, 3685–3709. [Google Scholar] [CrossRef]
- Sarıbaş, Ş.E.; Ulugöl, A. Struggle with bonzai: A review on synthetic cannabinoid abuse. Turk. Med Stud. J. 2014, 1, 86–93. [Google Scholar]
- Uchiyama, N.; Shimokawa, Y.; Matsuda, S.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. Two new synthetic cannabinoids, AM-2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1 H-indol-3-yl) methanone (MEPIRAPIM), and three phenethylamine derivatives, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and 2C-N-NBOMe, identified in illegal products. Forensic Toxicol. 2014, 32, 105–115. [Google Scholar]
- Schoeder, C.T.; Hess, C.; Madea, B.; Meiler, J.; Müller, C.E. Pharmacological evaluation of new constituents of “Spice”: Synthetic cannabinoids based on indole, indazole, benzimidazole and carbazole scaffolds. Forensic Toxicol. 2018, 36, 385–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochizuki, A.; Nakazawa, H.; Adachi, N.; Takekawa, K.; Shojo, H. Identification and quantification of mepirapim and acetyl fentanyl in authentic human whole blood and urine samples by GC-MS/MS and LC-MS/MS. Forensic Toxicol. 2018, 36, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Koeda, A.; Fujino, Y.; Onodera, M.; Kikuchi, S.; Niitsu, H.; Iwasaki, Y.; Usui, K.; Inoue, Y. Clinical and toxicological findings of acute intoxication with synthetic cannabinoids and cathinones. Acute Med. Surg. 2016, 3, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Leonhart, M. Schedules of Controlled Substances: Placement of Five Synthetic Cannabinoids into Schedule I; Federal Register; Drug Enforcement Administration: Springfield, VA, USA, 2012; pp. 12508–12514.
- Gardner, E.L. What we have learned about addiction from animal models of drug self-administration. Am. J. Addict. 2000, 9, 285–313. [Google Scholar] [CrossRef]
- Hur, K.-H.; Ma, S.-X.; Lee, B.-R.; Ko, Y.-H.; Seo, J.-Y.; Ryu, H.W.; Kim, H.J.; Yoon, S.; Lee, Y.-S.; Lee, S.-Y. Abuse potential of synthetic cannabinoids: AM-1248, CB-13, and PB-22. Biomol. Ther. 2021, 29, 384. [Google Scholar] [CrossRef]
- Tzschentke, T.M. Review on CPP: Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol. 2007, 12, 227–462. [Google Scholar] [CrossRef]
- Fattore, L.; Cossu, G.; Martellotta, C.M.; Fratta, W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology 2001, 156, 410–416. [Google Scholar] [CrossRef]
- De Luca, M.A.; Bimpisidis, Z.; Melis, M.; Marti, M.; Caboni, P.; Valentini, V.; Margiani, G.; Pintori, N.; Polis, I.; Marsicano, G. Stimulation of in vivo dopamine transmission and intravenous self-administration in rats and mice by JWH-018, a Spice cannabinoid. Neuropharmacology 2015, 99, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Cengel, H.Y.; Bozkurt, M.; Evren, C. The severity of non-planning impulsivity and cannabis use problems in distinguishing patients with synthetic cannabinoid use disorder and cannabis use disorder. Dusunen Adam 2021, 34, 41–49. [Google Scholar]
- Di Chiara, G.; Bassareo, V.; Fenu, S.; De Luca, M.A.; Spina, L.; Cadoni, C.; Acquas, E.; Carboni, E.; Valentini, V.; Lecca, D. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology 2004, 47, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.; Lapka, M.; Havlickova, T.; Syslova, K.; Sustkova-Fiserova, M. Alterations in rat accumbens dopamine, endocannabinoids and GABA content during WIN55, 212-2 treatment: The role of ghrelin. Int. J. Mol. Sci. 2021, 22, 210. [Google Scholar] [CrossRef] [PubMed]
- Pattij, T.; Vanderschuren, L.J.M.J. The neuropharmacology of impulsive behaviour. Trends Pharmacol. Sci. 2008, 29, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Braida, D.; Pozzi, M.; Cavallini, R.; Sala, M. Conditioned place preference induced by the cannabinoid agonist CP 55,940: Interaction with the opioid system. Neuroscience 2001, 104, 923–926. [Google Scholar] [CrossRef]
- Cha, H.J.; Lee, K.-W.; Song, M.-J.; Hyeon, Y.-J.; Hwang, J.-Y.; Jang, C.-G.; Ahn, J.-I.; Jeon, S.-H.; Kim, H.-U.; Kim, Y.-H. Dependence potential of the synthetic cannabinoids JWH-073, JWH-081, and JWH-210: In vivo and in vitro approaches. Biomol. Ther. 2014, 22, 363. [Google Scholar] [CrossRef] [Green Version]
- Owolabi, J.; Olatunji, S.; Olanrewaju, A. Caffeine and cannabis effects on vital neurotransmitters and enzymes in the brain tissue of juvenile experimental rats. Ann. Neurosci. 2017, 24, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Ossato, A.; Uccelli, L.; Bilel, S.; Canazza, I.; Di Domenico, G.; Pasquali, M.; Pupillo, G.; De Luca, M.A.; Boschi, A.; Vincenzi, F.; et al. Psychostimulant Effect of the Synthetic Cannabinoid JWH-018 and AKB48: Behavioral, Neurochemical, and Dopamine Transporter Scan Imaging Studies in Mice. Front. Psychiatry 2017, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Hur, K.H.; Kim, S.E.; Ma, S.X.; Lee, B.R.; Ko, Y.H.; Seo, J.Y.; Kim, S.K.; Kim, Y.J.; Sung, S.J.; Lee, Y. Methoxphenidine (MXP) induced abnormalities: Addictive and schizophrenia-related behaviours based on imbalance of neurochemicals in the brain. Br. J. Pharmacol. 2021, 178, 3869–3887. [Google Scholar] [CrossRef]
- Hur, K.H.; Kim, S.E.; Lee, B.R.; Ko, Y.H.; Seo, J.Y.; Kim, S.K.; Ma, S.X.; Kim, Y.J.; Jeong, Y.; Pham, D.T.; et al. 25C-NBF, a new psychoactive substance, has addictive and neurotoxic potential in rodents. Arch. Toxicol. 2020, 94, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Hur, K.H.; Ko, Y.H.; Kim, K.; Lee, B.R.; Kim, Y.J.; Kim, S.K.; Kim, S.E.; Lee, Y.S.; Kim, H.C.; et al. A novel designer drug, 25N-NBOMe, exhibits abuse potential via the dopaminergic system in rodents. Brain Res. Bull. 2019, 152, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Metna-Laurent, M.; Mondésir, M.; Grel, A.; Vallée, M.; Piazza, P.V. Cannabinoid-Induced Tetrad in Mice. Curr. Protoc. Neurosci. 2017, 80, 9.59.1–9.59.10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Galaj, E.; Bi, G.H.; Zhang, C.; He, Y.; Zhan, J.; Bauman, M.H.; Gardner, E.L. Different receptor mechanisms underlying phytocannabinoid- versus synthetic cannabinoid-induced tetrad effects: Opposite roles of CB(1)/CB(2) versus GPR55 receptors. Br. J. Pharmacol. 2020, 177, 1865–1880. [Google Scholar] [CrossRef]
- Zhao, R.J.; Yoon, S.S.; Lee, B.H.; Kwon, Y.K.; Kim, K.J.; Shim, I.; Choi, K.H.; Kim, M.R.; Golden, G.T.; Yang, C.H. Acupuncture normalizes the release of accumbal dopamine during the withdrawal period and after the ethanol challenge in chronic ethanol-treated rats. Neurosci. Lett. 2006, 395, 28–32. [Google Scholar] [CrossRef]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hur, K.-H.; Lee, Y.; Donio, A.L.; Ma, S.-X.; Lee, B.-R.; Kim, S.-K.; Lee, J.-G.; Kim, Y.-J.; Kim, M.; Yoon, S.; et al. Mepirapim, a Novel Synthetic Cannabinoid, Induces Addiction-Related Behaviors through Neurochemical Maladaptation in the Brain of Rodents. Pharmaceuticals 2022, 15, 710. https://doi.org/10.3390/ph15060710
Hur K-H, Lee Y, Donio AL, Ma S-X, Lee B-R, Kim S-K, Lee J-G, Kim Y-J, Kim M, Yoon S, et al. Mepirapim, a Novel Synthetic Cannabinoid, Induces Addiction-Related Behaviors through Neurochemical Maladaptation in the Brain of Rodents. Pharmaceuticals. 2022; 15(6):710. https://doi.org/10.3390/ph15060710
Chicago/Turabian StyleHur, Kwang-Hyun, YouYoung Lee, Audrey Lynn Donio, Shi-Xun Ma, Bo-Ram Lee, Seon-Kyung Kim, Jae-Gyeong Lee, Young-Jung Kim, MinJeong Kim, SeolMin Yoon, and et al. 2022. "Mepirapim, a Novel Synthetic Cannabinoid, Induces Addiction-Related Behaviors through Neurochemical Maladaptation in the Brain of Rodents" Pharmaceuticals 15, no. 6: 710. https://doi.org/10.3390/ph15060710