Iron Metabolism Dysregulation and Cognitive Dysfunction in Pediatric Obesity: Is There a Connection?
Abstract
:1. Introduction
2. Iron Homeostasis
3. Hepcidin, Master Regulator of Systemic Iron Homeostasis
4. Iron Deficiency in Obese Children

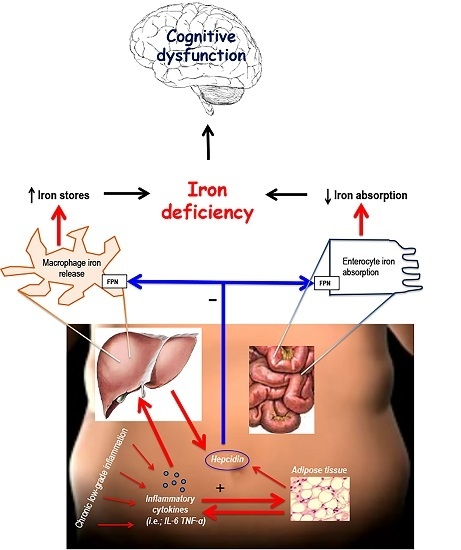
5. Proposed Mechanisms Linking Obesity and Iron Deficiency: The Role of Hepcidin

6. Iron Deficiency and Cognition
7. Obesity and Cognitive Function
8. Clinical Implications and Future Directions
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Santoro, N.; Cirillo, G.; Lepore, M.G.; Palma, A.; Amato, A.; Savarese, P.; Marzuillo, P.; Grandone, A.; Perrone, L.; del Giudice, E.M. Effect of the rs997509 polymorphism on the association between ectonucleotide pyrophosphatase phosphodiesterase 1 and metabolic syndrome and impaired glucose tolerance in childhood obesity. J. Clin. Endocrinol. Metab. 2009, 94, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Grandone, A.; Conte, M.; Capuano, F.; Cirillo, G.; di Sessa, A.; Umano, G.R.; Romano, R.; Perrone, L.; del Giudice, E.M. Novel association between a nonsynonymous variant (R270H) of the G-protein-coupled receptor 120 and liver injury in children and adolescents with obesity. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Grandone, A.; Perrone, L.; del Giudice, E.M. Weight loss allows the dissection of the interaction between abdominal fat and PNPLA3 (adiponutrin) in the liver damage of obese children. J. Hepatol. 2013, 59, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; del Giudice, E.M.; Santoro, N. Pediatric non-alcoholic fatty liver disease: New insights and future directions. World J. Hepatol. 2014, 6, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Jáuregui-Lobera, I. Iron deficiency and cognitive functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Jimenez, E.; Hagen, J.; Mollen, E.; Wolf, A.W. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 2000, 105, E51. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2015. [Google Scholar] [CrossRef]
- Bouglé, D.; Brouard, J. Iron in child obesity. Relationships with inflammation and metabolic risk factors. Nutrients 2013, 5, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, B.J.; Stults, H.B.; Mayer, J. Hypoferraemia in obese adolescents. Lancet 1962, 2, 327–328. [Google Scholar] [CrossRef]
- Adams, P.F.; Marano, M.A. Current estimates from the National Health Interview Survey, 1994. National Center for Health Statistics. Vital. Health Stat. 1995, 10, 193. [Google Scholar]
- Nead, K.G.; Halterman, J.S.; Kaczorowski, J.M.; Auinger, P.; Weitzman, M. Overweight children and adolescents: A risk group for iron deficiency. Pediatrics 2004, 114, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Newfield, R.S.; Koren, I.; Agmon, A.; Lilos, P.; Phillip, M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Gillum, R.F. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—The Third National Health and Nutrition Examination Survey. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Hurrell, R.F.; Zimmermann, M.B. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int. J. Obes. (Lond.) 2009, 33, 1111–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Matheson, B.E.; Kaye, W.H.; Boutelle, K.N. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int. J. Obes. (Lond.) 2014, 38, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Tussing-Humphreys, L.; Pusatcioglu, C.; Nemeth, E.; Braunschweig, C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: Introducing hepcidin. J. Acad. Nutr. Diet. 2012, 112, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.F.; Wessling-Resnick, M.; Knutson, M.D. Hepcidin regulation of iron transport. J. Nutr. 2008, 138, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Olbina, G.; Girelli, D.; Nemeth, E.; Westerman, M. Immunoassay for human serum hepcidin. Blood 2008, 112, 4292–4297. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Chauvet, C.; Viatte, L.; Danan, J.L.; Bigard, X.; Devaux, I.; Beaumont, C.; Kahn, A.; Vaulont, S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Investig. 2002, 110, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, D.A.; Roy, C.N.; Fleming, M.D.; Loda, M.F.; Wolfsdorf, J.I.; Andrews, N.C. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood 2002, 100, 3776–3781. [Google Scholar] [CrossRef] [PubMed]
- Tussing-Humphreys, L.; Frayn, K.N.; Smith, S.R.; Westerman, M.; Dennis, A.L.; Nemeth, E.; Thomson, J.; Pusatcioglu, C. Subcutaneous adipose tissue from obese and lean adults does not release hepcidin in vivo. Sci. World J. 2011, 11, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Nikonorov, A.A.; Skalnaya, M.G.; Tinkov, A.A.; Skalny, A.V. Mutual interaction between iron homeostasis and obesity pathogenesis. J. Trace Elem. Med. Biol. 2015, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hassapidou, M.; Fotiadou, E.; Magiara, E.; Papadopoulou, S.K. Energy intake, diet composition, energy expenditure, and body fatness of adolescents in northern Greece. Obesity 2006, 14, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Menzie, C.M.; Yanoff, L.B.; Denkinger, B.I.; McHugh, T.; Sebring, N.G.; Calis, K.A.; Yanovski, J.A. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J. Am. Diet. Assoc. 2008, 108, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; Aroche, C.; Plouffe, L.J.; Lee, M.; Murtaza, Z.; Kenney, L.; Lavergne, C.; Aziz, A. Diet-induced obese rats have higher iron requirements and are more vulnerable to iron deficiency. Eur. J. Nutr. 2014, 53, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Yanoff, L.B.; Menzie, C.M.; Denkinger, B.; Sebring, N.G.; McHugh, T.; Remaley, A.T.; Yanovski, J.A. Inflammation and iron deficiency in the hypoferremia of obesity. Int. J. Obes. (Lond.) 2007, 31, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.M.; Santoro, N.; Amato, A.; Brienza, C.; Calabrò, P.; Wiegerinck, E.T.; Cirillo, G.; Tartaglione, N.; Grandone, A.; Swinkels, D.W.; et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J. Clin. Endocrinol. Metab. 2009, 94, 5102–5107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, R.T.; Hamed, A.I.; Kharshoum, R.R. Iron homeostasis and serum hepcidin-25 levels in obese children and adolescents: Relation to body mass index. Horm. Res. Paediatr. 2013, 80, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.W.; Yang, Q.; Mody, N.; Graham, T.E.; Hsu, C.H.; Xu, Z.; Houstis, N.E.; Kahn, B.B.; Rosen, E.D. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007, 56, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Auguet, T.; Quintero, Y.; Terra, X.; Martínez, S.; Lucas, A.; Pellitero, S.; Aguilar, C.; Hernández, M.; del Castillo, D.; Richart, C. Upregulation of lipocalin 2 in adipose tissues of severely obese women: Positive relationship with proinflammatory cytokines. Obesity 2011, 19, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Akelma, A.Z.; Abaci, A.; Ozdemir, O.; Celik, A.; Avci, Z.; Razi, C.H.; Hizli, S.; Akin, O. The association of serum lipocalin-2 levels with metabolic and clinical parameters in obese children: A pilot study. J. Pediatr. Endocrinol. Metab. 2012, 25, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Navarro, B.; Matute, E.; Vásquez-Garibay, E.; Zarabozo, D. Effect of chronic iron deficiency on neuropsychological domains in infants. J. Child Neurol. 2012, 27, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. The metabolic tune-up: Metabolic harmony and disease prevention. J. Nutr. 2003, 133, 1544S–1548S. [Google Scholar] [PubMed]
- Beard, J. One person’s view of iron deficiency, development, and cognitive function. Am. J. Clin. Nutr. 1995, 62, 709–710. [Google Scholar] [PubMed]
- Kretchmer, N.; Beard, J.L.; Carlson, S. The role of nutrition in the development of normal cognition. Am. J. Clin. Nutr. 1996, 63, 997S–1001S. [Google Scholar] [PubMed]
- Grantham-McGregor, S.M.; Ani, C.C. The role of micronutrients in psychomotor and cognitive development. Br. Med. Bull. 1999, 55, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, E. The developmental and probabilistic nature of the functional consequences of iron-deficiency anemia in children. J. Nutr. 2001, 131, 669S–675S. [Google Scholar] [PubMed]
- Lozoff, B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J. Nutr. 2011, 141, 740S–746S. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, Q.; Jackson, J.C.; Zhang, J. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity 2008, 16, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Maayan, L.; Hoogendoorn, C.; Sweat, V.; Convit, A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity 2011, 19, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.L.; Cooper, S. Fitness, fatness, cognition, behavior, and academic achievement among overweight children: Do cross-sectional associations correspond to exercise trial outcomes? Prev. Med. 2011, 52, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.; Schmelter, A.; Kasten, L.; Heil, M. Impaired mental rotation performance in overweight children. Appetite 2011, 56, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Mond, J.M.; Stich, H.; Hay, P.J.; Kraemer, A.; Baune, B.T. Associations between obesity and developmental functioning in pre-school children: A population-based study. Int. J. Obes. (Lond.) 2007, 31, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Krombholz, H. The motor and cognitive development of overweight preschool children. Early Years 2012, 32, 61–70. [Google Scholar] [CrossRef]
- Cliff, D.P.; Okely, A.D.; Magarey, A.M. Movement skill mastery in a clinical sample of overweight and obese children. Int. J. Pediatr. Obes. 2011, 6, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Cliff, D.P.; Okely, A.D.; Morgan, P.J.; Jones, R.A.; Steele, J.R.; Baur, L.A. Proficiency deficiency: Mastery of fundamental movement skills and skill components in overweight and obese children. Obesity 2012, 20, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Morano, M.; Colella, D.; Caroli, M. Gross motor skill performance in a sample of overweight and non-overweight preschool children. Int. J. Pediatr. Obes. 2011, 6, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Okely, A.D.; Booth, M.L.; Chey, T. Relationships between body composition and fundamental movement skills among children and adolescents. Res. Q. Exerc. Sport 2004, 75, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Barrigas, C.; Fragoso, I. Obesity, academic performance and reasoning ability in Portuguese students between 6 and 12 years old. J. Biosoc. Sci. 2012, 44, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Yehuda, S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: Involvement of dopamine-opiate system. Cell Mol. Biol. 2000, 46, 491–500. [Google Scholar] [PubMed]
- Armony-Sivan, R.; Kaplan-Estrin, M.; Jacobson, S.W.; Lozoff, B. Iron-deficiency anemia in infancy and mother-infant interaction during feeding. J. Dev. Behav. Pediatr. 2010, 31, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Castillo, M.; Clark, K.M.; Smith, J.B.; Sturza, J. Iron supplementation in infancy contributes to more adaptive behavior at 10 years of age. J. Nutr. 2014, 144, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, S.; Yehuda, M. Long lasting effects of infancy iron deficiency—Preliminary results. J. Neural. Transm. Suppl. 2006, 71, 197–200. [Google Scholar] [PubMed]
- Sanad, M.; Osman, M.; Gharib, A. Obesity modulate serum hepcidin and treatment outcome of iron deficiency anemia in children: A case control study. Ital. J. Pediatr. 2011, 37, 34. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Zeder, C.; Muthayya, S.; Winichagoon, P.; Chaouki, N.; Aeberli, I.; Hurrell, R.F. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int. J. Obes. (Lond.) 2008, 32, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Santoro, N.; Calabrò, P.; Grandone, A.; Swinkels, D.W.; Perrone, L.; del Giudice, E.M. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int. J. Obes. (Lond.) 2010, 34, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; YIuan, F.; Teng, J.; Li, X.; Zheng, S.; Lin, L.; Deng, H.; Ma, G.; Sun, C.; Li, Y. Weight loss, inflammatory markers, and improvements of iron status in overweight and obese children. J. Pediatr. 2014, 164, 795–800. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandone, A.; Marzuillo, P.; Perrone, L.; Del Giudice, E.M. Iron Metabolism Dysregulation and Cognitive Dysfunction in Pediatric Obesity: Is There a Connection? Nutrients 2015, 7, 9163-9170. https://doi.org/10.3390/nu7115458
Grandone A, Marzuillo P, Perrone L, Del Giudice EM. Iron Metabolism Dysregulation and Cognitive Dysfunction in Pediatric Obesity: Is There a Connection? Nutrients. 2015; 7(11):9163-9170. https://doi.org/10.3390/nu7115458
Chicago/Turabian StyleGrandone, Anna, Pierluigi Marzuillo, Laura Perrone, and Emanuele Miraglia Del Giudice. 2015. "Iron Metabolism Dysregulation and Cognitive Dysfunction in Pediatric Obesity: Is There a Connection?" Nutrients 7, no. 11: 9163-9170. https://doi.org/10.3390/nu7115458