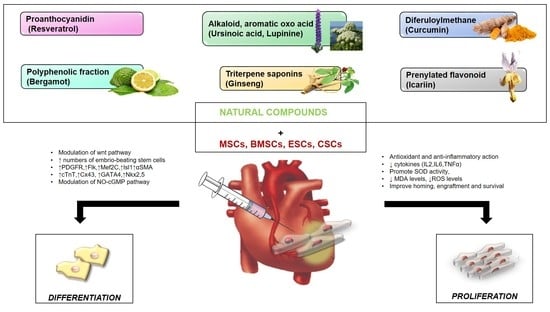
The Potential Properties of Natural Compounds in Cardiac Stem Cell Activation: Their Role in Myocardial Regeneration
Abstract
:1. Introduction
2. Cardiac Regeneration and Stem Cells Therapy
2.1. Embryonic Stem Cells
2.2. Induced Pluripotent Stem Cells
2.3. Bone Marrow Derived Cells
2.4. Mesenchimal Stem Cells
2.5. Cardiac Stem Cells
| Stem Cell Type | Properties | In Vitro/In Vivo Models | Clinical Trials | References |
|---|---|---|---|---|
| Embryonic stem cells (ESCs) |
|
|
| [24,71,74,75,76,79,82,85] |
| Induced Pluripotent stem cells (iPSCs) |
|
|
| [19,74,92,93,95,96,97] |
| Bone marrow derived cells (BMSCs) |
|
|
| [22,109,110,111,112] |
| Mesenchimal stem cells (MSCs) |
|
|
| [118,119,120,121,122,123,124,125] |
| Cardiac stem cells (CSCs) |
|
|
| [13,23,26,27,58,59,60,137] |
2.6. Mechanisms Involved in Cardiac Regeneration
3. Plant Extracts and Their Role in Nutraceutical Supplementation
Natural Compounds and Cardiovascular Protection
4. Natural Compounds and Stem Cells Activation and Differentiation in CVDs
4.1. Lupinine and Ursinoic Acid
4.2. Resveratrol
4.3. Ginseng
4.4. Icariin
4.5. Curcumin
4.6. BPF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs). Available online: http://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases (accessed on 1 June 2019).
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. Executive Summary: Heart Disease and Stroke Statistics—2015 Update. A Report from the American Heart Association. Circulation 2015, 131, 434–441. [Google Scholar] [CrossRef]
- Fuster, V.; Kelly, B.B. (Eds.) Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries. In Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Cesselli, D.; Aleksova, A.; Mazzega, E.; Caragnano, A.; Beltrami, A.P. Cardiac stem cell aging and heart failure. Pharmacol. Res. 2018, 127, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Epidemiology of Cardiovascular Disease. Available online: https://www.nature.com/collections/bedbejdbij?proof=t (accessed on 20 November 2020).
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakboonyarat, B.; Rangsin, R. Prevalence and associated factors of ischemic heart disease (IHD) among patients with diabetes mellitus: A nationwide, cross-sectional survey. BMC Cardiovasc. Disord. 2018, 18, 151. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Lu, K.; Zhu, J.; Wang, J. Stem cell therapy for ischemic heart diseases. Br. Med. Bull. 2017, 121, 135–154. [Google Scholar] [CrossRef] [Green Version]
- Terashvili, M.; Bosnjak, Z.J. Stem Cell Therapies in Cardiovascular Disease. J. Cardiothorac. Vasc. Anesth. 2019, 33, 209–222. [Google Scholar] [CrossRef]
- Hsieh, P.C.; Segers, V.F.; Davis, M.E.; MacGillivray, C.; Gannon, J.; Molkentin, J.D.; Robbins, J.; Lee, R.T. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat. Med. 2007, 13, 970–974. [Google Scholar] [CrossRef]
- Sun, R.; Li, X.; Liu, M.; Zeng, Y.; Chen, S.; Zhang, P. Advances in Stem Cell Therapy For Cardiovascular Disease (Review). Int. J. Mol. Med. 2016, 38, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Wernly, B.; Mirna, M.; Rezar, R.; Prodinger, C.; Jung, C.; Podesser, B.K.; Kiss, A.; Hoppe, U.C.; Lichtenauer, M. Regenerative Cardiovascular Therapies: Stem Cells and Beyond. Int. J. Mol. Sci. 2019, 20, 1420. [Google Scholar] [CrossRef] [Green Version]
- Ménard, C.; Hagège, A.A.; Agbulut, O.; Barro, M.; Morichetti, M.C.; Brasselet, C.; Bel, A.; Messas, E.; Bissery, A.; Bruneval, P.; et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: A preclinical study. Lancet 2005, 366, 1005–1012. [Google Scholar] [CrossRef]
- Sartiani, L.; Bettiol, E.; Stillitano, F.; Mugelli, A.; Cerbai, E.; Jaconi, M.E. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: A molecular and electrophysiological approach. Stem Cells 2007, 25, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Ethical and regulatory aspects of embryonic stem cell research. Expert Opin. Biol. Ther. 2005, 5, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.V.; Kensah, G.; Rotaermel, A.; Baraki, H.; Kutschka, I.; Zweigerdt, R.; Martin, U.; Haverich, A.; Gruh, I.; Martens, A. Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction. PLoS ONE 2017, 12, e0173222. [Google Scholar] [CrossRef] [PubMed]
- Riggs, J.W.; Barrilleaux, B.L.; Varlakhanova, N.; Bush, K.M.; Chan, V.; Knoepfler, P.S. Induced pluripotency and oncogenic transformation are related processes. Stem Cells Dev. 2012, 22, 37–50. [Google Scholar] [CrossRef]
- Yoshihara, M.; Hayashizaki, Y.; Murakawa, Y. Genomic instability of iPSCs: Challenges towards their clinical applications. Stem Cell Rev. Rep. 2017, 13, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Strauer, B.E.; Brehm, M.; Zeus, T.; Kostering, M.; Hernandez, A.; Sorg, R.V.; Kögler, G.; Wernet, P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002, 106, 1913–1918. [Google Scholar] [CrossRef] [Green Version]
- Ellison, G.M.; Vicinanza, C.; Smith, A.J.; Aquila, I.; Leone, A.; Waring, C.D.; Henning, B.J.; Stirparo, G.G.; Papait, R.; Scarfò, M.; et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 2013, 154, 827–842. [Google Scholar] [CrossRef] [Green Version]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef] [Green Version]
- Musunuru, K.; Sheikh, F.; Gupta, R.M.; Houser, S.R.; Maher, K.O.; Milan, D.J.; Terzic, A.; Wu, J.C. Induced pluripotent stem cells for cardiovascular disease modeling and precision medicine: A scientific statement from the American Heart Association. Circ. Genom. Precis Med. 2018, 11, e000043. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marban, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, T.; Makkar, R.R.; Ascheim, D.D.; Traverse, J.H.; Schatz, R.; DeMaria, A.; Francis, G.S.; Povsic, T.J.; Smith, R.R.; Lima, J.A.; et al. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) Trial: Rationale and Design. Cell Transplant. 2017, 26, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Kroess, R.; Bonaros, N.; Marksteiner, R.; Margreiter, E.; Schachner, T.; Laufer, G.; Hering, S. Intramyocardial microdepot injection increases the efficacy of skeletal myoblast transplantation. Eur. J. Cardiothorac. Surg. 2005, 27, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.F.; Tirouvanziam, A.M.; Perigaud, C.; Fernandes, S.; Fusellier, M.S.; Desfontis, J.C.; Toquet, C.S.; Heymann, M.F.; Crochet, D.P.; Lemarchand, P.F. Cell distribution after intracoronary bone marrow stem cell delivery in damaged and undamaged myocardium: Implications for clinical trials. Stem Cell Res. Ther. 2010, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Aicher, A.; Brenner, W.; Zuhayra, M.; Badorff, C.; Massoudi, S.; Assmus, B.; Eckey, T.; Henze, E.; Zeiher, A.M.; Dimmeler, S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 2003, 107, 2134–2139. [Google Scholar] [CrossRef] [Green Version]
- Miyagawa, S.; Saito, A.; Sakaguchi, T.; Yoshikawa, Y.; Yamauchi, T.; Imanishi, Y.; Kawaguchi, N.; Teramoto, N.; Matsuura, N.; Iida, H.; et al. Impaired myocardium regeneration with skeletal cell sheets—A preclinical trial for tissue-engineered regeneration therapy. Transplantation 2010, 90, 364–372. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Ma, X.; Adila, A.; Wang, B.; Liu, F.; Chen, B.; Wang, C.; Ma, Y. Preservation of the cardiac function in infarcted rat hearts by the transplantation of adipose-derived stem cells with injectable fibrin scaffolds. Exp. Biol. Med. (Maywood) 2010, 235, 1505–1515. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, Y.S.; Ahn, Y.; Jeong, M.H.; Hong, M.H.; Joo, S.Y.; Nam, K.I.; Cho, J.G.; Kang, P.M.; Park, J.C. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc. Res. 2006, 70, 530–542. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Hu, X.; Fu, W.; Xie, J.; Zhou, X.; Xu, W.; Jiang, H. Dobutamine-mediated heme oxygenase-1 induction via PI3K and p38 MAPK inhibits high mobility group box 1 protein release and attenuates rat myocardial ischemia/reperfusion injury in vivo. J. Surg. Res. 2013, 183, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xia, X.; Tao, Q.; Lu, K.; Shen, J.; Xu, Q.; Hu, X.; Tang, Y.; Block, N.L.; Webster, K.A.; et al. Profound actions of an agonist of growth hormone-releasing hormone on angiogenic therapy by mesenchymal stem cells. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 663–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Wei, L.; Taylor, T.M.; Wei, J.; Zhou, X.; Wang, J.A.; Yu, S.P. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via kv2.1 channel and FAK activation. Am. J. Physiol. Cell Physiol. 2011, 301, C362–C372. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, Z.; Hu, S.; Chen, H.; Cong, X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J. Cell Biochem. 2010, 111, 967–978. [Google Scholar] [CrossRef]
- Huang, K.; Hu, S.; Cheng, K. A New Era of Cardiac Cell Therapy: Opportunities and Challenges. Adv. Healthc. Mater. 2019, 8, e1801011. [Google Scholar] [CrossRef]
- Rikhtegar, R.; Pezeshkian, M.; Dolati, S.; Safaie, N.; Afrasiabi Rad, A.; Mahdipour, M.; Nouri, M.; Jodati, A.R.; Yousefi, M. Stem cells as therapy for heart disease: iPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed. Pharmacother. 2019, 109, 304–313. [Google Scholar] [CrossRef]
- Wang, J.A.; Li, C.L.; Fan, Y.Q.; He, H.; Sun, Y. Allograftic bone marrow-derived mesenchymal stem cells transplanted into heart infarcted model of rabbit to renovate infarcted heart. J. Zhejiang. Univ. Sci. 2004, 5, 1279–1285. [Google Scholar] [CrossRef] [Green Version]
- Ventura, C.; Cavallini, C.; Bianchi, F.; Cantoni, S. Stem Cells and Cardiovascular Repair: A Role for Natural and Synthetic Molecules Harboring Differentiating and Paracrine Logics. Cardiovasc. Hematol. Agents. Med. Chem. 2008, 6, 60–68. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef]
- Uemura, R.; Xu, M.; Ahmad, N.; Ashraf, M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ. Res. 2006, 98, 1414–1421. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Fan, G.C. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 138–149. [Google Scholar] [PubMed]
- Sluijter, J.P.; Verhage, V.; Deddens, J.C.; van den Akker, F.; Doevendans, P.A. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate pi3k/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noiseux, N.; Borie, M.; Desnoyers, A.; Menaouar, A.; Stevens, L.M.; Mansour, S.; Danalache, B.A.; Roy, D.C.; Jankowski, M.; Gutkowska, J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology 2012, 153, 5361–5372. [Google Scholar] [CrossRef] [PubMed]
- Wisel, S.; Khan, M.; Kuppusamy, M.L.; Mohan, I.K.; Chacko, S.M.; Rivera, B.K.; Sun, B.C.; Hideg, K.; Kuppusamy, P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-2,3,4-trimethoxybenzylpiperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J. Pharmacol. Exp. Ther. 2009, 329, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Hoke, N.N.; Salloum, F.N.; Kass, D.A.; Das, A.; Kukreja, R.C. Preconditioning by phosphodiesterase-5 inhibition improves therapeutic efficacy of adipose-derived stem cells following myocardial infarction in mice. Stem Cells 2012, 30, 326–335. [Google Scholar] [CrossRef]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and improvement Strategies. Cell Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction—From repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef]
- Turan, R.D.; Aslan, G.S.; Yücel, D.; Döğer, R.; Kocabaş, F. Evolving approaches to heart regeneration by therapeutic stimulation of resident cardiomyocyte cell cycle. Anatol. J. Cardiol. 2016, 16, 881–886. [Google Scholar] [PubMed]
- Zhao, X.; Huang, I. Cardiac stem cells in patients with heart disease. Exp. Ther. Med. 2013, 5, 1273–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laflamme, M.A.; Murry, C.E. Heart Regeneration. Nature 2011, 473, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anversa, P.; Leri, A.; Kajstura, J.; Nadal-Ginard, B. Myocyte Growth and Cardiac Repair. J. Mol. Cell Cardiol. 2002, 34, 91–105. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef] [Green Version]
- Barile, L.; Messina, E.; Giacomello, A.; Marbán, E. Endogenous Cardiac Stem Cells. Progr. Cardiovasc. Dis. 2007, 50, 31–48. [Google Scholar] [CrossRef]
- Buja, L.M. Cardiac repair and the putative role of stem cells. J. Mol. Cell. Cardiol. 2019, 128, 96–104. [Google Scholar] [CrossRef]
- Biehl, J.K.; Russell, B. Introduction to Stem Cell Therapy. J. Cardiovasc. Nurs. 2009, 24, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Leong, Y.Y.; Ng, W.H.; Ellison- Hughes, G.M.; Tan, J.J. Cardiac Stem Cells for Myocardial Regeneration: They Are Not Alone. Front. Cardiovasc. Med. 2017, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Lal, S.; Le, T.Y.L.; dos Remedios, C.; Chong, J.J.H. Cardiac stem cells: Translation to human studies. Biophys. Rev. 2015, 7, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Rota, M.; Padin-Iruegas, M.E.; Misao, Y.; De Angelis, A.; Maestroni, S.; Ferreira-Martins, J.; Fiumana, E.; Rastaldo, R.; Arcarese, M.L.; Mitchell, T.S.; et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ. Res. 2008, 103, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; Lecapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oettgen, P.; Boyle, A.; Schulman, S.P.; Hare, J.M. Cardiac Stem Cell Therapy. Need for Optimization of Efficacy and Safety Monitoring. Circulation 2006, 114, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.L.; Rokosh, G.; Sanganalmath, S.K.; Yuan, F.; Sato, H.; Mu, J.; Dai, S.; Li, C.; Chen, N.; Peng, Y.; et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 2010, 121, 293–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J. Cell. Biochem. 2017, 119, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Myerson, D.; Saffitz, J.E.; Murry, C.E. Evidence for Cardiomyocyte Repopulation by Extracardiac Progenitors in Transplanted Human Hearts. Circ. Res. 2002, 90, 634–640. [Google Scholar] [CrossRef] [Green Version]
- du Pré, B.C.; Doevendans, P.A.; van Laake, L.W. Stem cells for cardiac repair: An introduction. J. Geriatr. Cardiol. 2013, 10, 186−197. [Google Scholar]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [Green Version]
- Samak, M.; Hinkel, R. Stem Cells in Cardiovascular Medicine: Historical Overview and Future Prospects. Cells 2019, 8, 1530. [Google Scholar] [CrossRef] [Green Version]
- Duelen, R.; Sampaolesi, M. Stem Cell Technology in Cardiac Regeneration: A Pluripotent Stem Cell Promise. EBioMedicine 2017, 16, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Leri, A.; Kajstura, J.; Anversa, P. Cardiac Stem Cells and Mechanisms of Myocardial Regeneration. Physiol. Rev. 2005, 85, 1373–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henning, R.J. Current status of stem cells in cardiac repair. Future Cardiol. 2018, 14, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Kenyagin-Karsenti, D.; Snir, M.; Segev, H.; Amit, M.; Gepstein, A.; Livne, E.; Binah, O.; Itskovitz-Eldor, J.; Gepstein, L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Investig. 2001, 108, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Ardehali, R.; Ali, S.R.; Inlay, M.A.; Abilez, O.J.; Chen, M.Q.; Blauwkamp, T.A.; Yazawa, M.; Gong, Y.; Nusse, R.; Drukker, M.; et al. Prospective isolation of human embryonic stem cell-derived cardiovascular progenitors that integrate into human fetal heart tissue. Proc. Natl. Acad. Sci. USA 2013, 110, 3405–3410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, D.K. Embryonic Stem Cells in Cardiac Repair and Regeneration. Antioxid. Redox Signal 2009, 11, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lian, X.L. Heart regeneration with human pluripotent stem cells: Prospects and challenges. Bioact. Mater. 2020, 5, 74–81. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human ESC-Derived Cardiomyocytes Restore Function in Infarcted Hearts of Non-Human Primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef]
- Nouspikel, T. Genetic instability in human embryonic stem cells: Prospects and caveats. Future Oncol. 2013, 9, 867–877. [Google Scholar] [CrossRef]
- Cohen, I.G.; Adashi, E.Y. Human embryonic stem-cell research under siege—Battle won but not the war. N. Engl. J. Med. 2011, 364, e48. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.; Clement, K.; Mallard, W.; Tagliazucchi, G.M.; Lim, H.; Choi, I.Y.; Ferrari, F.; Tsankov, A.; Pop, R.; et al. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nat. Biotechnol. 2015, 33, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Boue, S.; Izpisua Belmonte, J.C. Methods for making induced pluripotent stem cells: Reprogramming a la carte. Nat. Rev. Genet. 2011, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Laugwitz, K.L.; Dorn, T.; Sinnecker, D.; Mummery, C. Pluripotent stem cell models of human heart disease. Cold Spring Harb. Perspect. Med. 2013, 3, a014027. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamazaki, T.; El Rouby, N.; Fredette, N.C.; Santostefano, K.E.; Terada, N. Concise Review: Induced Pluripotent Stem Cell Research in the Era of Precision Medicine. Stem Cells. 2017, 35, 545–550.3de. [Google Scholar] [CrossRef] [Green Version]
- Mauritz, C.; Schwanke, K.; Reppel, M. Generation of Functional Murine Cardiac Myocytes from Induced Pluripotent Stem Cells. Circulation 2008, 118, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xi, Y.; Zheng, Y.; Wang, X.; Cooney, A.J. Generation of Electrophysiologically Functional Cardiomyocytes from Mouse Induced Pluripotent Stem Cells. Stem Cell Res. 2016, 16, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.; Huber, B.C.; Lee, W.H.; Kodo, K.; Ebert, A.D.; Ma, Y.; Nguyen, P.K.; Diecke, S.; Chen, W.Y.; Wu, J.C. Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation 2015, 132, 762–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakikes, I.; Ameen, M.; Termglinchan, V.; Wu, J.C. Human induced pluripotent stem cell-derived cardiomyocytes: Insights into molecular, cellular, and functional phenotypes. Circ. Res. 2015, 117, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsa, E.; Burridge, P.W.; Wu, J.C. Human stem cells for modeling heart disease and for drug discovery. Sci. Transl. Med. 2014, 6, 239ps6. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.; Chao, B.S.; Wu, J.C. Strategies for Improving the Maturity of Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Circ. Res. 2018, 123, 512–514. [Google Scholar] [CrossRef]
- Goversen, B.; van der Heyden, M.A.G.; van Veen, T.A.B.; de Boer, T.P. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on IK1. Pharmacol. Ther. 2018, 183, 127–136. [Google Scholar] [CrossRef]
- Machiraju, P.; Greenway, S.C. Current methods for the maturation of induced pluripotent stem cell-derived cardiomyocytes. World J. Stem Cells 2019, 11, 33–43. [Google Scholar] [CrossRef]
- Parikh, S.S.; Blackwell, D.J.; Gomez-Hurtado, N.; Frisk, M.; Wang, L.; Kim, K.; Dahl, C.P.; Fiane, A.; Tønnessen, T.; Kryshtal, D.O.; et al. Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Res. 2017, 121, 1323–1330. [Google Scholar] [CrossRef]
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef]
- Cui, Z.; Ni, N.C.; Wu, J.; Du, G.Q.; He, S.; Yau, T.M.; Weisel, R.D.; Sung, H.W.; Li, R.K. Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 2018, 8, 2752–2764. [Google Scholar] [CrossRef]
- Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Carido, M.; Sebastião, M.J.; Gomes-Alves, P.; Elliott, D.A.; Domian, I.J.; Teixeira, A.P.; et al. 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes. Biotechnol. Bioeng. 2018, 115, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behfar, A.; Crespo-Diaz, R.; Terzic, A.; Gersh, B.J. Cell therapy for cardiac repair--lessons from clinical trials. Nat. Rev. Cardiol. 2014, 11, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Dawn, B.; Bolli, R. Adult bone marrow-derived cells: Regenerative potential, plasticity, and tissue commitment. Basic Res. Cardiol. 2005, 100, 494–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef]
- Czarna, A.; Sanada, F.; Matsuda, A.; Kim, J.; Signore, S.; Pereira, J.D.; Sorrentino, A.; Kannappan, R.; Cannatà, A.; Hosoda, T.; et al. Single-cell analysis of the fate of c-kit-positive bone marrow cells. NPJ Regen. Med. 2017, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Jeevanantham, V.; Butler, M.; Saad, A.; Abdel-Latif, A.; Zuba-Surma, E.K.; Dawn, B. Adult Bone Marrow Cell Therapy Improves Survival and Induces Long-Term Improvement in Cardiac Parameters: A Systematic Review and Meta-Analysis. Circulation 2012, 126, 551–568. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.R.; Samanta, A.; Shah, Z.I.; Jeevanantham, V.; Abdel-Latif, A.; Zuba-Surma, E.K.; Dawn, B. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease: Evidence and Insights from Randomized Controlled Trials. Circ. Res. 2015, 117, 558–575. [Google Scholar] [CrossRef] [Green Version]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Chugh, A.; Yang, P.C.; Zhao, D.X.M.; Ellis, S.G.; Forder, J.R.; Perin, E.C.; et al. TIME Trial-Effect of Timing of Stem Cell Delivery Following ST-Elevation Myocardial Infarction on the Recovery of Global and Regional Left Ventricular Function: Final 2-Year Analysis. Circ. Res. 2018, 122, 479–488. [Google Scholar] [CrossRef]
- Martino, H.; Brofman, P.; Greco, O.; Bueno, R.; Bodanese, L.; Clausell, N.; Maldonado, J.A.; Mill, J.; Braile, D.; Moraes, J., Jr.; et al. Multicentre, randomized, double-blind trial of intracoronary autologous mononuclear bone marrow cell injection in non-ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study). Eur. Heart J. 2015, 36, 2898–2904. [Google Scholar] [CrossRef] [Green Version]
- Balsam, L.B.; Wagers, A.J.; Christensen, J.L.; Kofidis, T.; Weissman, I.L.; Robbins, R.C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004, 428, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amado, L.C.; Saliaris, A.P.; Schuleri, K.H.; St John, M.; Xie, J.S.; Cattaneo, S.; Durand, D.J.; Fitton, T.; Kuang, J.Q.; Stewart, G.; et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA 2005, 102, 11474–11479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, M.X.; He, A.N.; Wang, J.A.; Gui, C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J. Zhejiang Univ. Sci. B 2009, 10, 619–624. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Thakker, R.; Yang, P. Mesenchymal Stem Cell Therapy for Cardiac Repair. Curr Treat. Options Cardiovasc. Med. 2014, 16, 323. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Huang, Y.; Chen, Z.; Xia, Y.; Chen, A.; Lu, D.; Wu, Y.; Zhang, N.; Qian, J. Efficacy of mesenchymal stem cell therapy in systolic heart failure: A systematic review and meta-analysis. Stem Cell Res. Ther. 2019, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Van Linthout, S.; Stamm, C.; Schultheiss, H.P.; Tschöpe, C. Mesenchymal Stem Cells and Inflammatory Cardiomyopathy: Cardiac Homing and Beyond. Cardiol. Res. Pract. 2011, 2011, 757154. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res. Ther. 2017, 8, 242. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Singh, A.; Sen, D. Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010–2015). Stem Cell Res. Ther. 2016, 7, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrami, A.; Urbanek, K.; Kajstura, J.; Yan, S.M.; Finato, N.; Bussani, R.; Nadal-Ginard, B.; Silvestri, F.; Leri, A.; Beltrami, C.A.; et al. Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 2001, 344, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, K.; Quaini, F.; Tasca, G.; Torella, D.; Castaldo, C.; Nadal-Ginard, B.; Leri, A.; Kajstura, J.; Quaini, E.; Anversa, P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2003, 100, 10440–10445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef] [Green Version]
- Anversa, P.; Nadal-Ginard, B. Myocyte renewal and ventricular remodeling. Nature 2002, 415, 240–243. [Google Scholar] [CrossRef]
- Quaini, F.; Urbanek, K.; Beltrami, A.P.; Finato, N.; Beltrami, C.A.; Nadal-Ginard, B.; Kajstura, J.; Leri, A.; Anversa, P. Chimerism of the transplanted heart. N. Engl. J. Med. 2002, 346, 5–15. [Google Scholar] [CrossRef]
- Hong, K.U.; Bolli, R. Cardiac stem cell therapy for cardiac repair. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R.; Tang, X.L.; Sanganalmath, S.K.; Rimoldi, O.; Mosna, F.; Abdel-Latif, A.; Jneid, H.; Rota, M.; Leri, A.; Kajstura, J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 2013, 128, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Ellison, G.M.; Torella, D.; Dellegrottaglie, S.; Perez-Martinez, C.; Perez de Prado, A.; Vicinanza, C.; Purushothaman, S.; Galuppo, V.; Iaconetti, C.; Waring, C.D.; et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J. Am. Coll Cardiol. 2011, 58, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Vicinanza, C.; Aquila, I.; Scalise, M.; Cristiano, F.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Sacco, W.; Lewis, F.C.; et al. Adult cardiac stem cells are multipotent and robustly myogenic: C-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017, 24, 2101–2116. [Google Scholar] [CrossRef] [Green Version]
- Chugh, A.R.; Beache, G.M.; Loughran, J.H.; Mewton, N.; Elmore, J.B.; Kajstura, J.; Pappas, P.; Tatooles, A.; Stoddard, M.F.; Lima, J.A.; et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The SCIPIO trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 2012, 126, S54–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, S.; Ohtsuki, S.; Eitoku, T.; Ousaka, D.; Kondo, M.; Kurita, Y.; Hirai, K.; Fukushima, Y.; Baba, K.; Goto, T.; et al. Intracoronary Cardiac Progenitor Cells in Single Ventricle Physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ. Res. 2017, 120, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Fleischmann, B.K.; Kotlikoff, M.I. Concise review: The role of C-kit expressing cells in heart repair at the neonatal and adult stage. Stem Cells 2014, 32, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Jesty, S.A.; Steffey, M.A.; Lee, F.K.; Breitbach, M.; Hesse, M.; Reining, S.; Lee, J.C.; Doran, R.M.; Nikitin, A.Y.; Fleischmann, B.K.; et al. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA 2012, 109, 13380–13385. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Ginard, B.; Ellison, G.M.; Torella, D. The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult. Stem Cell Res. 2014, 13, 615–630. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Ginard, B.; Torella, D.; De Angelis, A.; Rossi, F. Monographic issue of pharmacological research on adult myocardial repair/regeneration. Pharmacol. Res. 2018, 127, 1–3. [Google Scholar] [CrossRef]
- Khodayari, S.; Khodayari, H.; Amiri, A.Z.; Eslami, M.; Farhud, D.; Hescheler, J.; Nayernia, K. Inflammatory Microenvironment of +Acute Myocardial Infarction Prevents Regeneration of Heart with Stem Cells Therapy. Cell Physiol. Biochem. 2019, 53, 887–909. [Google Scholar]
- Abdelwahid, E.; Kalvelyte, A.; Stulpinas, A.; de Carvalho, K.A.; Guarita-Souza, L.C.; Foldes, G. Stem cell death and survival in heart regeneration and repair. Apoptosis 2016, 21, 252–268. [Google Scholar] [CrossRef] [Green Version]
- De Bakker, B.S.; de Jong, K.H.; Hagoort, J.; de Bree, K.; Besselink, C.T.; de Kanter, F.E.; Veldhuis, T.; Bais, B.; Schildmeijer, R.; Ruijter, J.M.; et al. An interactive three-dimensional digital atlas and quantitative database of human development. Science 2016, 354, aag0053. [Google Scholar] [CrossRef]
- Fan, Y.; Ho, B.X.; Pang, J.K.S.; Pek, N.M.Q.; Hor, J.H.; Ng, S.Y.; Soh, B.S. Wnt/β-catenin-mediated signaling re-activates proliferation of matured cardiomyocytes. Stem Cell Res. Ther. 2018, 9, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Pan, W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozhan, G.; Weidinger, G. Wnt/β-catenin signaling in heart regeneration. Cell Regen. 2015, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, Y.; Yang, Y.; Li, Q.; He, X.; Zhu, W.; Wang, J.; Gan, X. Oligomerization of Frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/β-catenin pathway. J. Biol. Chem. 2018, 293, 19710–19724. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Pan, B.; Zhou, H.; Liu, L.; Lv, T.; Zhu, J.; Huang, X.; Tian, J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J. Biomed. Sci. 2017, 24, 29. [Google Scholar] [CrossRef]
- Fu, J.D.; Rushing, S.N.; Lieu, D.K.; Chan, C.W.; Kong, C.W.; Geng, L.; Wilson, K.D.; Chiamvimonvat, N.; Boheler, K.R.; Wu, J.C.; et al. Distinct roles of microRNA-1and -499 in ventricular specification and functional maturation of human embryonic stem cells-derived cardiomyocytes. PLoS ONE 2011, 6, e27417. [Google Scholar] [CrossRef] [Green Version]
- Marino, F.; Scalise, M.; Cianflone, E.; Mancuso, T.; Aquila, I.; Agosti, V.; Torella, M.; Paolino, D.; Mollace, V.; Nadal-Ginard, B.; et al. Role of c-Kit in Myocardial Regeneration and Aging. Front. Endocrinol. 2019, 10, 371. [Google Scholar] [CrossRef]
- Der Sarkissian, S.; Lévesque, T.; Noiseux, N. Optimizing stem cells for cardiac repair: Current status and new frontiers in regenerative cardiology. World J. Stem Cells 2017, 9, 9–25. [Google Scholar] [CrossRef]
- Hodgkinson, C.P.; Bareja, A.; Gomez, J.A.; Dzau, V.J. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ. Res. 2016, 118, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Li, T.S.; Suzuki, R.; Kobayashi, T.; Ito, H.; Ikeda, Y.; Matsuzaki, M.; Hamano, K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H886–H893. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Joo, H.W.; Park, I.H.; Shen, G.Y.; Lee, Y.; Shin, J.H.; Kim, H.; Kim, K.S. Bone marrow mesenchymal stem cell-derived vascular endothelial growth factor attenuates cardiac apoptosis via regulation of cardiac miRNA-23a and miRNA-92a in a rat model of myocardial infarction. PLoS ONE 2017, 12, e0179972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Du, W.; Liu, J.; Ma, W.; Zhang, L.; Du, Z.; Cai, B. Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. Front. Pharmacol. 2018, 9, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Li, Q.; Xu, J.; Xu, J.; Xiong, Y.; Chen, G.; Qian, H.; Jin, C.; Yu, Y.; et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res. Ther. 2019, 10, 300. [Google Scholar] [CrossRef] [Green Version]
- Davidson, S.M.; Takov, K.; Yellon, D.M. Exosomes and Cardiovascular Protection. Cardiovasc. Drugs Ther. 2017, 31, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef]
- Giricz, Z.; Varga, Z.V.; Baranyai, T.; Sipos, P.; Pálóczi, K.; Kittel, Á.; Buzás, E.I.; Ferdinandy, P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J. Mol. Cardiol. 2014, 68, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Rohailla, S.; Gelber, N.; Rutka, J.; Sabah, N.; Gladstone, R.A.; Wei, C.; Hu, P.; Kharbanda, R.K.; Redington, A.N. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res. Cardiol. 2014, 109, 423. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Li, N.; Duan, C.E.; Yang, Y.J. Cardiac progenitor/stem cells on myocardial infarction or ischemic heart disease: What we have known from current research. Heart Fail. Rev. 2014, 19, 247–258. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K. Traditional herbs: A remedy for cardiovascular disorders. Phytomedicine 2016, 23, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Salas, J.; Vazquez, F.M.; Hortigón-Vinagre, M.P.; Ruiz-Tellez, T. Bioactive Phytochemicals from Mercurialis spp. Used in Traditional Spanish Medicine. Plants 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halberstein, R.A. Medicinal plants: Historical and cross-cultural usage patterns. Ann. Epidemiol. 2005, 15, 686–699. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ji, G.; Zhu, L. Editorial: Herbal Medicine on High Impact Disease: The Current Progress and Application. Curr. Pharmac. Des. 2017, 23. [Google Scholar] [CrossRef]
- Shukla, S.K.; Gupta, S.; Ojha, S.K.; Sharma, S.B. Cardiovascular friendly natural products: A promising approach in the management of CVD. Nat. Prod. Res. 2010, 24, 873–898. [Google Scholar] [CrossRef]
- Micucci, M.; Malaguti, M.; Toschi, T.G.; Di Lecce, G.; Aldini, R.; Angeletti, A.; Chiarini, A.; Budriesi, R.; Hrelia, S. Cardiac and Vascular Synergic Protective Effect of Olea europea L. Leaves and Hibiscus sabdariffa L. Flower Extracts. Oxid. Med. Cell Longev. 2015, 2015, 318125. [Google Scholar] [CrossRef] [Green Version]
- Arauna, D.; Furrianca, M.; Espinosa-Parrilla, Y.; Fuentes, E.; Alarcón, M.; Palomo, I. Natural Bioactive Compounds as Protectors Of Mitochondrial Dysfunction In Cardiovascular Diseases And Aging. Molecules 2019, 24, 4259. [Google Scholar] [CrossRef] [Green Version]
- Kinder, D.H.; Knecht, K.T. Lupine (Lupinus caudatus L., Lupinus albus L.) Seeds: History of Use, Use as an Antihyperglycemic Medicinal, and Use as a Food. Nuts Seeds Health Dis. Prev. 2011, 711–716. [Google Scholar] [CrossRef]
- Knecht, K.T.; Nguyen, H.; Auker, A.D.; Kinder, D.H. Effects of extracts of lupine seed on blood glucose levels in glucose resistant mice: Antihyperglycemic effects of Lupinus albus (white lupine, Egypt) and Lupinus caudatus (tailcup lupine, Mesa Verde National Park. J. Herb. Pharmacother. 2006, 6, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Belski, R.; Mori, T.A.; Puddey, I.B.; Sipsas, S.; Woodman, R.J.; Ackland, T.R.; Beilin, L.J.; Dove, E.R.; Carlyon, N.B.; Jayaseena, V.; et al. Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: A 12-month randomized controlled weight loss trial. Int. J. Obes. 2011, 35, 810–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Zhang, Y. Icariin, an Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed. Front. Pharmacol. 2017, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Wang, Z.; Shi, J. Pharmacological effects of icariin. Adv. Pharmacol. 2020, 87, 179–203. [Google Scholar]
- Zhao, F.; Tang, Y.Z.; Liu, Z.Q. Protective effect of icariin on DNA against radical-induced oxidative damage. J. Pharm. Pharmacol. 2007, 59, 1729–1732. [Google Scholar] [CrossRef]
- Song, Y.H.; Cai, H.; Zhao, Z.M.; Chang, W.J.; Gu, N.; Cao, S.P.; Wu, M.L. Icariin attenuated oxidative stress induced-cardiac apoptosis by mitochondria protection and ERK activation. Biomed. Pharmacother. 2016, 83, 1089–1094. [Google Scholar] [CrossRef]
- Chen, S.R.; Xu, X.Z.; Wang, Y.H.; Chen, J.W.; Xu, S.W.; Gu, L.Q.; Liu, P.Q. Icariin derivative inhibits inflammation through suppression of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways. Biol. Pharm. Bull. 2010, 33, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Sun, B.; Liu, K.; Yan, M.; Zhang, Y.; Miao, C.; Ren, L. Icariin attenuates high-cholesterol diet induced atherosclerosis in rats by inhibition of inflammatory response and p38 MAPK signaling pathway. Inflammation 2016, 39, 228–236. [Google Scholar] [CrossRef]
- Xiao-Hong, D.; Chang-Qin, X.; Jian-Hua, H.; Wen-Jiang, Z.; Bing, S. Icariin delays homocysteine-induced endothelial cellular senescence involving activation of the PI3K/AKT-eNOS signaling pathway. Pharm. Biol. 2013, 51, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Ke, Z.; Liu, J.; Xu, P.; Gao, A.; Wang, L.; Ji, L. The Cardioprotective Effect of Icariin on Ischemia-Reperfusion Injury in Isolated Rat Heart: Potential Involvement of the PI3K-Akt Signaling Pathway. Cardiovasc. Ther. 2015, 33, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, T.; Lin, N.; Huang, X.; Lu, W.; Sun, Z.; Zhang, J.; Lin, H.; Chi, J.; Guo, H. Icariin Ameliorates Diabetic Cardiomyopathy Through Apelin/Sirt3 Signalling to Improve Mitochondrial Dysfunction. Front. Pharmacol. 2020, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Cai, H.; Gu, N.; Qian, C.F.; Cao, S.P.; Zhao, Z.M. Icariin attenuates cardiac remodelling through down-regulating myocardial apoptosis and matrix metalloproteinase activity in rats with congestive heart failure. J. Pharm Pharmacol. 2011, 63, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, C.; Zhang, Y.; Wu, X.D. Ginseng: An Nonnegligible Natural Remedy for Healthy Aging. Aging Dis. 2017, 8, 708–720. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H. Cardiovascular Diseases and Panax ginseng: A Review on Molecular Mechanisms and Medical Applications. J. Ginseng Res. 2012, 36, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.D. Use of Ginseng in Medicine with Emphasis on Neurodegenerative Disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, T.; Kim, S.W.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012, 32, 718–726. [Google Scholar] [CrossRef]
- Liu, L.; Kelly, M.G.; Wierzbicki, E.L.; Escober-Nario, I.C.; Vollmer, M.K.; Doré, S. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J. Ethnopharmacol. 2010, 130, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.Q.; Li, T.; Wang, J.R.; Wong, V.K.; Luo, P.; Wong, I.Y.; Jiang, Z.H.; Liu, L.; Zhou, H. Total ginsenosides increase coronary perfusion flow in isolated rat hearts through activation of PI3K/Akt-eNOS signaling. Phytomedicine 2010, 17, 1006–1015. [Google Scholar] [CrossRef]
- Tsutsumi, Y.M.; Tsutsumi, R.; Mawatari, K.; Nakaya, Y.; Kinoshita, M.; Tanaka, K.; Oshita, S. Compound K, a metabolite of ginsenosides, induces cardiac protection mediated nitric oxide via Akt/PI3K pathway. Life Sci. 2011, 88, 725–729. [Google Scholar] [CrossRef]
- Lim, K.H.; Ko, D.; Kim, J.H. Cardioprotective potential of Korean Red Ginseng extract on isoproterenol-induced cardiac injury in rats. J. Ginseng Res. 2013, 37, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.P.; Jiang, Y.C.; Yu, X.F.; Xu, H.L.; Li, M.; Zhao, X.Z.; Sui, D.Y. Ginsenoside Rg3 Improves Cardiac Function after Myocardial ischemia/Reperfusion via Attenuating Apoptosis and Inflammation. Evid. Based Complement. Alternat. Med. 2016, 2016, 6967853. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.M.; Hong, S.J.; Choi, S.C.; Park, J.H.; Kim, J.S.; Lim, D.S. Red ginseng extract improves coronary flow reserve and increases absolute numbers of various circulating angiogenic cells in patients with first ST-segment elevation acute myocardial infarction. Phytother. Res. 2011, 25, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice. From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef]
- Wongcharoen, W.; Phrommintikul, A. The protective role of curcumin in cardiovascular diseases. Int. J. Cardiol. 2009, 133, 145–151. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Tang, L.; Pan, Y.; Liu, Z.; Zeng, C.; Wang, J.; Wei, T.; Liang, G. Novel curcumin analogue 14p protects against myocardial ischemia reperfusion injury through Nrf2-activating anti-oxidative activity. Toxicol. Appl. Pharmacol. 2015, 282, 175–183. [Google Scholar] [CrossRef]
- Tian, K.; Ogura, S.; Little, P.J.; Xu, S.W.; Sawamura, T. Targeting LOX-1 in atherosclerosis and vasculopathy: Current knowledge and future perspectives. Ann. N. Y. Acad. Sci. 2019, 1443, 34–53. [Google Scholar] [CrossRef]
- Thompson, J.W.; Wei, J.; Appau, K.; Wang, H.; Yu, H.; Spiga, M.G.; Graham, R.M.; Webster, K.A. Bnip3 binds and activates p300: Possible role in cardiac transcription and myocyte morphology. PLoS ONE 2015, 10, e0136847. [Google Scholar] [CrossRef] [Green Version]
- Rahnavard, M.; Hassanpour, M.; Ahmadi, M.; Heidarzadeh, M.; Amini, H.; Javanmard, M.Z.; Nouri, M.; Rahbarghazi, R.; Safaie, N. Curcumin ameliorated myocardial infarction by inhibition of cardiotoxicity in the rat model. J. Cell Biochem. 2019. [CrossRef] [PubMed]
- Wang, N.P.; Wang, Z.F.; Tootle, S.; Philip, T.; Zhao, Z.Q. Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br. J. Pharmacol. 2012, 167, 1550–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, C.W.; Yoo, K.Y.; Lee, S.H.; Jeong, H.J.; Lee, C.S.; Kim, S.J. Curcumin protects against regional myocardial ischemia/reperfusion injury through activation of RISK/GSK-3β and inhibition of p38 MAPK and JNK. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Qiao, Z.; Xu, Y. Protective effect of curcumin against myocardium injury in ischemia reperfusion rats. Pharm. Biol. 2017, 55, 1144–1148. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Das, D.K. Grapes, Wines, Resveratrol, and Heart Health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [Green Version]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef]
- Gliozzi, M.; Carresi, C.; Musolino, V.; Palma, E.; Muscoli, C.; Vitale, C.; Gratteri, S.; Muscianisi, G.; Janda, E.; Muscoli, S.; et al. The effect of bergamot-derived polyphenolic fraction on LDL small dense particles and non-alcoholic fatty liver disease in patients with metabolic syndrome. Adv. Biol. Chem. 2014, 4, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Musolino, V.; Gliozzi, M.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; Scicchitano, M.; et al. The effect of bergamot polyphenolic fraction on lipid transfer protein system and vascular oxidative stress in a rat model of hyperlipemia. Lipids Health Dis. 2019, 18, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Parafati, M.; Lascala, A.; Morittu, V.M.; Trimboli, F.; Rizzuto, A.; Brunelli, E.; Coscarelli, F.; Costa, N.; Britti, D.; Ehrlich, J.; et al. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J. Nutr. Biochem. 2015, 26, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot polyphenols improve dyslipidemia and pathophysiological features in a Mouse Model of non-Alcoholic fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and ckitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef]
- La Russa, D.; Giordano, F.; Marrone, A.; Parafati, M.; Janda, E.; Pellegrino, D. Oxidative Imbalance and Kidney Damage in Cafeteria Diet-Induced Rat Model of Metabolic Syndrome: Effect of Bergamot Polyphenolic Fraction. Antioxidants 2019, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in nonalcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Nikonov, G.K.; Yagudaev, M.R. Structure of ursinoic acid and ursinin. Chem. Nat. Comp. 1970, 6, 429–432. [Google Scholar] [CrossRef]
- Lee, J.A.; An, J.; Kang, T.M.; De, D.; Kim, K.K. Discovery of Natural Compounds Promoting Cardiomyocyte Differentiation. Stem Cells Dev. 2019, 28, 13–27. [Google Scholar] [CrossRef]
- Gurusamy, N.; Ray, D.; Lekli, I.; Das, D.K. Red wine antioxidant resveratrol-modified cardiac stem cells regenerate infarcted myocardium. J. Cell. Mol. Med. 2010, 14, 2235–2239. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.; Gu, S.; Cheng, Y. Resveratrol activates endogenous cardiac stem cells and improves myocardial regeneration following acute myocardial infarction. Mol. Med. Rep. 2017, 15, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Gorbunov, N.; Petrovski, G.; Gurusamy, N.; Ray, D.; Kim, D.H.; Das, D.K. Regeneration of infarcted myocardium with resveratrol-modified cardiac stem cells. J. Cell. Mol. Med. 2012, 16, 174–184. [Google Scholar] [CrossRef] [Green Version]
- ShamsEldeen, A.M.; Ashour, H.; Shoukry, H.S.; Fadel, M.; Kamar, S.S.; Aabdelbaset, M.; Rashed, L.A.; Ammar, H.I. Combined treatment with systemic resveratrol and resveratrol preconditioned mesenchymal stem cells, maximizes antifibrotic action in diabetic cardiomyopathy. J. Cell. Physiol. 2019, 234, 10942–10963. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Zhao, L.; Zhang, Y.; Li, Q.; Chai, X.; Zhang, Y. Resveratrol Enhances Cardiomyocyte Differentiation of Human Induced Pluripotent Stem Cells through Inhibiting Canonical WNT Signal Pathway and Enhancing Serum Response Factor-miR-1 Axis. Stem Cells Int. 2016, 2016, 2524092. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Xu, X.; Qin, X.; Yang, C.; Feng, W. Resveratrol promotes differentiation of mouse embryonic stem cells to cardiomyocytes. Cardiovasc. Ther. 2016, 34, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinarli, F.A.; Turan, N.N.; Pinarli, F.G.; Okur, A.; Sonmez, D.; Ulus, T.; Oguz, A.; Karadeniz, C.; Delibasi, T. Resveratrol and Adipose-derived Mesenchymal Stem Cells Are Effective in the Prevention and Treatment of Doxorubicin Cardiotoxicity in Rats. Pediatr. Hematol. Oncol. 2013, 30, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. The application of resveratrol to mesenchymal stromal cell-based regenerative medicine. Stem Cell Res. Ther. 2019, 10, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Jing, P.; Wang, L.; Zhang, Y.; Yong, J.; Wang, Y. The positive effects of Ginsenoside Rg1 upon the hematopoietic microenvironment in a D-Galactose-induced aged rat model. BMC Complement. Altern. Med. 2015, 15, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, L.S.; Yue, P.Y.K.; Mak, N.K.; Wong, R.N.S. Role of MicroRNA-214 in ginsenoside-Rg1-induced angiogenesis. Eur. J. Pharm. Sci. 2009, 38, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, R.; Liu, D.; Deng, W.; Xu, G.; Liu, W.; Rong, J.; Long, X.; Ge, J.; Shi, B. Exosomes Derived from miR-214-Enriched Bone Marrow-Derived Mesenchymal Stem Cells Regulate Oxidative Damage in Cardiac Stem Cells by Targeting CaMKII. Oxid. Med. Cell. Longev. 2018, 2018, 4971261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Ku, S.Y.; Rosenwaks, Z.; Liu, H.C.; Oh, S.K.; Moon, S.Y.; Choi, Y.M. Red Ginseng Extract Facilitates the Early Differentiation of Human Embryonic Stem Cells into Mesendoderm Lineage. Evid. Based Complement. Alternat. Med. 2011, 2011, 167376. [Google Scholar] [CrossRef]
- Sasaki, T.; Oh, K.B.; Matsuoka, H.; Saito, M. Effect of Panax ginseng components on the differentiation of mouse embryonic stem cells into cardiac-like cells. Yakugaku Zasshi 2008, 128, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.S.; Yue, P.Y.; Wong, Y.Y.; Wong, R.N. MicroRNA-15b contributes to ginsenoside-Rg1-induced angiogenesis through increased expression of VEGFR-2. Biochem. Pharmacol. 2013, 86, 392–400. [Google Scholar] [CrossRef]
- He, F.; Yu, C.; Liu, T.; Jia, H. Ginsenoside Rg1 as an Effective Regulator of Mesenchymal Stem Cells. Front. Pharmacol. 2020, 10, 1565. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Chen, G.; Chen, C.; Zhang, Y.; Yan, C. Effect of Ginsenoside Rg1 in promoting myocardial regeneration after myocardial infarction in rats (in Chinese). Chin. Heart. J. 2008, 20, 697–707. [Google Scholar]
- Kim, A.R.; Kim, S.W.; Lee, B.W.; Kim, K.H.; Kim, W.H.; Kim, W.H.; Seok, H.; Lee, J.H.; Um, J.; Yim, S.H.; et al. Screening ginseng saponins in progenitor cells identifies 20(R)-ginsenoside Rh2 as an enhancer of skeletal and cardiac muscle regeneration. Sci. Rep. 2020, 10, 4967. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Lou, Y.J. Inducible effects of icariin, icaritin, and desmethylicaritin on directional differentiation of embryonic stem cells into cardiomyocytes in vitro1. Acta Pharmacol. Sin. 2005, 26, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Qu, L.; Zhang, X.; Lou, Y. Icariin-mediated modulation of cell cycle and p53 during cardiomyocyte differentiation in embryonic stem cells. Eur. J. Pharmacol. 2005, 514, 99–110. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Lou, Y.J. Icariin-mediated expression of cardiac genes and modulation of nitric oxide signaling pathway during differentiation of mouse embryonic stem cells into cardiomyocytes in vitro1. Acta Pharmacol. Sin. 2006, 27, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Wo, Y.; Zhu, D.; Hu, Y.; Wang, Z.Q.; Liu, J.; Lou, Y.J. Reactive oxygen species involved in prenylflavonoids, icariin and icaritin, initiating cardiac differentiation of mouse embryonic stem cells. J. Cell. Biochem. 2008, 103, 1536–1550. [Google Scholar] [CrossRef]
- Jiang, C.; Gong, F. BMP-2 and icariin synergistically promote p38MAPK-mediated cardiomyocyte differentiation of mesenchymal stem cells via enhanced NOX4-driven ROS generation. Medic. Chem. Res. 2017, 26, 2547–2556. [Google Scholar] [CrossRef]
- Jin, M.S.; Shi, S.; Zhang, Y.; Yan, Y.; Sun, X.D.; Liu, W.; Liu, H.W. Icariin-mediated differentiation of mouse adipose-derived stem cells into cardiomyocytes. Mol. Cell. Biochem. 2010, 344, 1–9. [Google Scholar] [CrossRef]
- Backs, J.; Olson, E.N. Control of cardiac growth by histone acetylation/deacetylation. Circ. Res. 2006, 98, 15–24. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, J.; Lu, T.; Liu, L.; Sun, H.; Liu, Z.; Tian, J. p300-Mediated Histone Acetylation is Essential for the Regulation of GATA4 and MEF2C by BMP2 in H9c2 Cells. Cardiovasc. Toxicol. 2013, 13, 316–322. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Pan, B.; Zhu, J.; Huang, G.; Huang, X.; Tian, J. Inhibition of histone acetylation by curcumin reduces alcohol-induced expression of heart development-related transcription factors in cardiac progenitor cells. Biochem. Biophys. Res. Commun. 2012, 424, 593–596. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, P.; Song, P.; Xiong, W.; Chen, H.; Peng, W.; Wang, S.; Li, S.; Fu, Z.; Wang, Y.; et al. Pretreatment of Adipose Derived Stem Cells with Curcumin Facilitates Myocardial Recovery via Antiapoptosis and Angiogenesis. Stem Cells Int. 2015, 2015, 638153. [Google Scholar] [CrossRef] [Green Version]
- Mujoo, K.; Nikonoff, L.E.; Sharin, V.G.; Bryan, N.S.; Kots, A.Y.; Murad, F. Curcumin induces differentiation of embryonic stem cells through possible modulation of nitric oxide-cyclic GMP pathway. Protein Cell 2012, 3, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Y.; Yang, Y.; Zhang, W.; Luo, L.; Han, F.; Guan, H.; Tao, K.; Hu, D. Curcumin pretreatment protects against hypoxia/reoxgenation injury via improvement of mitochondrial function, destabilization of HIF-1α and activation of Epac1-Akt pathway in rat bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2019, 109, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.G.; Wang, X.M.; Ma, L.F.; Zhang, Y.M. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem. Biol. Interact. 2015, 242, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ring, A.; Kim, Y.M.; Kahn, M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev. 2014, 10, 512–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, F.; Zhang, W.; Liu, J. Membrane estrogen receptor alpha is an important modulator of bone marrow c-Kit+ cells mediated cardiac repair after myocardial infarction. Int. J. Clin. Exp. Pathol. 2015, 8, 4284–4295. [Google Scholar] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carresi, C.; Scicchitano, M.; Scarano, F.; Macrì, R.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.C.; Mollace, R.; Guarnieri, L.; et al. The Potential Properties of Natural Compounds in Cardiac Stem Cell Activation: Their Role in Myocardial Regeneration. Nutrients 2021, 13, 275. https://doi.org/10.3390/nu13010275
Carresi C, Scicchitano M, Scarano F, Macrì R, Bosco F, Nucera S, Ruga S, Zito MC, Mollace R, Guarnieri L, et al. The Potential Properties of Natural Compounds in Cardiac Stem Cell Activation: Their Role in Myocardial Regeneration. Nutrients. 2021; 13(1):275. https://doi.org/10.3390/nu13010275
Chicago/Turabian StyleCarresi, Cristina, Miriam Scicchitano, Federica Scarano, Roberta Macrì, Francesca Bosco, Saverio Nucera, Stefano Ruga, Maria Caterina Zito, Rocco Mollace, Lorenza Guarnieri, and et al. 2021. "The Potential Properties of Natural Compounds in Cardiac Stem Cell Activation: Their Role in Myocardial Regeneration" Nutrients 13, no. 1: 275. https://doi.org/10.3390/nu13010275