Impact of Graphene Work Function on the Electronic Structures at the Interface between Graphene and Organic Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of NH2-SAM and F-SAM on SiO2
2.2. Graphene Growth and Transfer
2.3. Characterizations
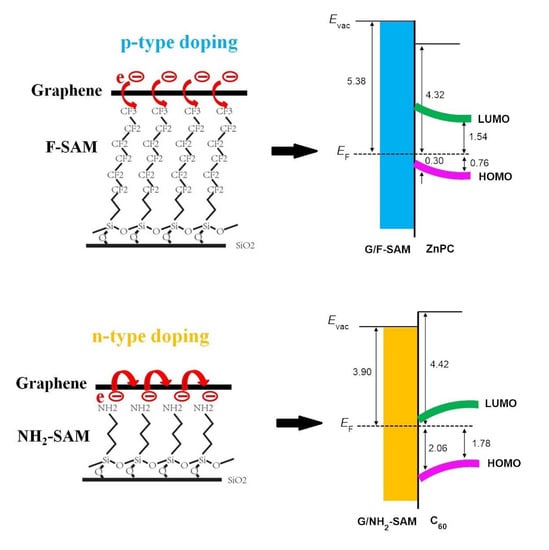
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706. [Google Scholar] [CrossRef] [PubMed]
- Ang, P.K.; Chen, W.; Wee, A.T.S.; Loh, K.P. Solution-gated epitaxial graphene as pH sensor. J. Am. Chem. Soc. 2008, 130, 14392. [Google Scholar] [CrossRef] [PubMed]
- Di, C.A.; Wei, D.C.; Yu, G.; Liu, Y.Q.; Guo, Y.L.; Zhu, D.B. Patterned graphene as source/drain electrodes for bottom-contact organic field-effect transistors. Adv. Mater. 2008, 20, 3289. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, D.Y.; Lee, S.J. Low-power graphene/ZnO schottky UV photodiodes with enhanced lateral schottky barrier homogeneity. Nanomaterials 2019, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Agrawal, M.; Becerril, H.A.; Bao, Z.N.; Liu, Z.F.; Chen, Y.S.; Peumans, P. Organic light-emitting diodes on solution-processed graphene transparent electrodes. ACS Nano 2010, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.P.; Hernandez, Y.; Feng, X.L.; Müllen, K. Graphene as transparent electrode material for organic electronics. Adv. Mater. 2011, 23, 2779. [Google Scholar] [CrossRef]
- Hasan, N.; Zhang, W.L.; Radadia, A.D. Few-flakes reduced graphene oxide sensors for organic vapors with a high signal-to-noise ratio. Nanomaterials 2017, 7, 339. [Google Scholar] [CrossRef]
- Xu, Y.F.; Long, G.L.; Huang, L.; Huang, Y.; Wan, X.J.; Ma, Y.F.; Chen, Y.S. Polymer photovoltaic devices with transparent graphene electrodes produced by spin-cast. Carbon 2010, 48, 3308. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, S.W.; Xu, X.F.; Özyilmaz, B.; Loh, K.P. Interface engineering of layer-by-layer stacked graphene anodes for high-performance organic solar cells. Adv. Mater. 2011, 23, 1514. [Google Scholar] [CrossRef]
- Luo, Q.; Ma, H.; Hou, Q.Z.; Li, Y.X.; Ren, J.; Dai, X.Z.; Yao, Z.B.; Zhou, Y.; Xiang, L.C.; Du, H.Y.; et al. All-carbon-electrode-based endurable flexible perovskite solar cells. Adv. Mater. 2018, 28, 1706777. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, H.J.; Yang, Q.L.; Xiong, C.X.; Li, W.; Yan, Y.; Gurney, R.S.; Wang, T. Solution-processed Graphene-MoS2 heterostructure for efficient hole extraction in organic solar cells. Carbon 2019, 142, 156. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Li, J.; Yang, J.P.; Cheng, P.P.; Zhao, J.; Lee, S.T.; Li, Y.Q.; Tang, J.X. Enhanced performance in polymer photovoltaic cells with chloroform treated indium tin oxide modification. Appl. Phys. Lett. 2011, 98, 253303. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-level alignment at organic/metal and organic/organic interface. Adv. Mater. 2009, 21, 1450. [Google Scholar] [CrossRef]
- Fukagawa, H.; Kera, S.; Kataoka, T.; Hosoumi, S.; Watanabe, Y.; Kudo, K.; Ueno, N. The role of the ionization potential in vacuum-level alignment at organic semiconductor interfaces. Adv. Mater. 2007, 19, 665. [Google Scholar] [CrossRef]
- Vázquez, H.; Oszwaldowski, R.; Pou, P.; Ortega, J.; Pérez, R.; Flores, F.; Kahn, A. Dipole formation at metal/PTCDA interfaces: Role of the charge neutrality level. Europhys. Lett. 2004, 65, 802. [Google Scholar] [CrossRef]
- Koch, N. Organic electronic devices and their functional interfaces. Chem. Phys. Chem. 2007, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.R.; Wang, W.N.; Alloway, D.M.; Placencia, D.; Ratcliff, E.; Brumbach, M. Organic/organic’ heterojunctions: Organic light emitting diodes and organic photovoltaic devices. Macromol. Rapid Commun. 2009, 30, 717. [Google Scholar] [CrossRef]
- Jesuraj, P.J.; Parameshwari, R.; Kanthasamy, K.; Koch, J.; Pfnür, H.; Jeganathan, K. Hole injection enhancement in organic light emitting devices using plasma treated graphene oxide. Appl. Surf. Sci. 2017, 397, 144. [Google Scholar] [CrossRef]
- Pandey, R.; Lim, J.W.; Kim, J.H.; Angadi, B.; Choi, J.W.; Choi, W.K. Performance enhancement in organic photovoltaic solar cells using iridium (Ir) ultrathin surface modifier (USM). Appl. Surf. Sci. 2018, 444, 97. [Google Scholar] [CrossRef]
- Dou, W.D.; Huang, S.P.; Lee, C.S. Graphene-enhanced intermolecular interaction at interface between copper- and cobalt-phthalocyanines. J. Chem. Phys. 2015, 143, 134706. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.D.; Huang, S.P.; Zhang, R.Q.; Lee, C.S. Molecular-substrate interaction channels of metal-phthalocyanines on graphene on Ni (111) surface. J. Chem. Phys. 2011, 134, 094705. [Google Scholar] [CrossRef] [PubMed]
- Akaike, K.; Nardi, M.V.; Oehzelt, M.; Frisch, J.; Opitz, A.; Christodoulou, C.; Ligorio, G.; Beyer, P.; Timpel, M.; Pis, I.; et al. Effective work function reduction of practical electrodes using an organometallic dimer. Adv. Mater. 2016, 26, 2493. [Google Scholar] [CrossRef]
- Helander, M.G.; Wang, Z.B.; Qiu, J.; Greiner, M.T.; Puzzo, D.P.; Liu, Z.W.; Lu, Z.H. Chlorinated indium tin oxide electrodes with high work function for organic device compatibility. Science 2011, 332, 944. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Amy, F.; Kahn, A. Spectroscopic study on sputtered PEDOT:PSS: Role of surface PSS layer. Org. Electron. 2006, 7, 387. [Google Scholar] [CrossRef]
- KrÖger, M.; Hamwi, S.; Meyer, J.; Riedl, T.; Lowalsky, W.; Kahn, A. Role of the deep-lying electronic states of MoO3 in the enhancement of hole-injection in organic thin films. Appl. Phys. Lett. 2009, 95, 123301. [Google Scholar] [CrossRef]
- Wu, Q.H.; Zhao, Y.Q.; Hong, G.; Ren, J.G.; Wang, C.D.; Zhang, W.J.; Lee, S.T. Electronic structure of MoO3-x/graphene interface. Carbon 2013, 65, 46. [Google Scholar] [CrossRef]
- Kim, D.Y.; Subbiah, J.; Sarasqueta, G.; So, F.; Ding, H.J.; Irfan; Gao, Y.L. The effect of molybdenum oxide interlayer on organic photovoltaic cells. Appl. Phys. Lett. 2009, 95, 093304. [Google Scholar] [CrossRef]
- Brabec, C.J.; Shaheen, S.E.; Winder, C.; Sariciftci, N.S. Effect of LiF/metal electrodes on the performance of plastic solar cells. Appl. Phys. Lett. 2002, 80, 1288. [Google Scholar] [CrossRef]
- Huang, J.S.; Xu, Z.; Yang, Y. Low-work-function surface formed by solution-processed and thermally deposited nanoscale layers of cesium carbonate. Adv. Funct. Mater. 2007, 17, 1966. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Yang, J.P.; Sun, F.Z.; Lee, S.T.; Li, Y.Q.; Tang, J.X. Efficient inverted polymer solar cells incorporating doped organic electron transporting layer. Org. Electron. 2012, 13, 697. [Google Scholar] [CrossRef]
- Mao, H.Y.; Hu, F.; Ye, Q.L.; Xu, Y.F.; Yang, X.X.; Lu, B. Manipulating the charge transfer at CuPc/graphene interface by O2 plasma treatments. Nanoscale 2014, 6, 8149. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Kang, B.H.; Lee, Y.D.; Lee, S.B.; Ju, B.K. Effect of fluorine plasma treatment with chemically reduced graphene oxide thin films as hole transport layer in organic solar cells. Appl. Surf. Sci. 2013, 287, 91. [Google Scholar] [CrossRef]
- Sherpa, S.D.; Levitin, G.; Hess, D.W. Effect of the polarity of carbon-fluorine bonds on the work function of plasma-fluorinated epitaxial graphene. Appl. Phys. Lett. 2012, 101, 111602. [Google Scholar] [CrossRef]
- Mao, H.Y.; Wang, R.; Wang, Y.; Niu, T.C.; Zhong, J.Q.; Huang, M.Y.; Qi, D.C.; Loh, K.P.; Wee, A.T.S.; Chen, W. Chemical vapor deposition graphene as structural template to control interfacial molecular orientation of chloroaluminium phthalocyanine. Appl. Phys. Lett. 2011, 99, 093301. [Google Scholar]
- Duhm, S.; Heimel, G.; Salzmann, I.; Glowatzki, H.; Johnson, R.L.; Vollmer, A.; Rabe, J.P.; Koch, N. Orientation-dependent ionization energies and interface dipoles in ordered molecular assemblies. Nat. Mater. 2008, 7, 326. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamada, K.; Tsutsumi, J.; Sato, N. Complete description of ionization energy and electron affinity in organic solids: Determining contributions from electronic polarization, energy band dispersion, and molecular orientation. Phys. Rev. B 2015, 92, 075145. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Qi, D.C.; Huang, H.; Gao, X.Y.; Wee, A.T.S. Organic-organic heterojunction interfaces: Effect of molecular orientation. Adv. Funct. Mater. 2011, 21, 410. [Google Scholar] [CrossRef]
- Chen, W.; Qi, D.C.; Huang, Y.L.; Huang, H.; Wang, Y.Z.; Chen, S.; Gao, X.Y.; Wee, A.T.S. Molecular orientation dependent energy level alignment at organic-organic heterojunction interfaces. J. Phys. Chem. C 2009, 113, 12832. [Google Scholar] [CrossRef]
- Schwarze, M.; Schellhammer, K.S.; Ortstein, K.; Benduhn, J.; Gaul, C.; Hinderhofer, A.; Toro, L.P.; Scholz, R.; Kublitski, J.; Roland, S.; et al. Impact of molecular quadrupole moments on the energy levels at organic heterojunctions. Nat. Commun. 2019, 10, 2466. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.Z.; Lu, W.; Yao, J.; Zhu, Y.; Tour, J.M. Controlled modulation of electronic properties of graphene by self-assembled monolayers on SiO2 substrtates. ACS Nano 2011, 5, 1535. [Google Scholar] [CrossRef]
- Park, J.; Jo, S.B.; Yu, Y.J.; Kim, Y.; Yang, J.W.; Lee, W.H.; Kim, H.H.; Hong, B.H.; Kim, P.; Cho, K.; et al. Single-gate bandgap opening of bilayer graphene by dual molecular doping. Adv. Mater. 2012, 24, 407. [Google Scholar] [CrossRef]
- Cao, Q.J.; Shi, B.Y.; Dou, W.D.; Tang, J.X.; Mao, H.Y. Background pressure does matter for the growth of graphene single crystal on copper foil: Key roles of oxygen partial pressure. Carbon 2018, 138, 458. [Google Scholar] [CrossRef]
- Satoh, T.; Imanishi, M.; Nishikawa, T.; Mori, T. Properties of interface between organic hole-transporting layer and indium tin oxide anode modified by fluorinated self-assembled monolayer. Jpn. J. Appl. Phys. 2012, 51, 035701. [Google Scholar] [CrossRef]
- Yang, Q.D.; Dou, W.D.; Wang, C.D.; Mo, H.W.; Lo, M.F.; Yuen, M.F.; Ng, T.W.; Zhang, W.J.; Tsang, S.W.; Lee, C.S. Effects of graphene defect on electronic structures of its interface with organic semiconductor. Appl. Phys. Lett. 2015, 106, 133502. [Google Scholar] [CrossRef]
- Dong, X.C.; Fu, D.L.; Fang, W.J.; Shi, Y.M.; Chen, P.; Li, L.J. Doping single-layer graphene with aromatic molecules. Small 2009, 5, 1422. [Google Scholar] [CrossRef]
- Mao, H.Y.; Bussolotti, F.; Qi, D.C.; Wang, R.; Kera, S.; Ueno, N.; Wee, A.T.S.; Chen, W. Mechanism of the Fermi level pinning at organic donor-acceptor heterojunction interfaces. Org. Electron. 2011, 12, 534. [Google Scholar] [CrossRef]
- Niederhausen, J.; Amsalem, P.; Wilke, A.; Schlesinger, R.; Winkler, S.; Vollmer, A.; Rabe, J.P.; Koch, N. Doping of C60 (sub)monolayers by Fermi-level pinning induced electron transfer. Phys. Rev. B 2012, 86, 081411. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, X.; Dou, W.; Wang, P.; Ye, Q.; Yang, X.; Li, B.; Mao, H. Impact of Graphene Work Function on the Electronic Structures at the Interface between Graphene and Organic Molecules. Nanomaterials 2019, 9, 1136. https://doi.org/10.3390/nano9081136
Wang H, Yang X, Dou W, Wang P, Ye Q, Yang X, Li B, Mao H. Impact of Graphene Work Function on the Electronic Structures at the Interface between Graphene and Organic Molecules. Nanomaterials. 2019; 9(8):1136. https://doi.org/10.3390/nano9081136
Chicago/Turabian StyleWang, Haitao, Xiangdong Yang, Weidong Dou, Peng Wang, Quanlin Ye, Xuxin Yang, Baoxing Li, and Hongying Mao. 2019. "Impact of Graphene Work Function on the Electronic Structures at the Interface between Graphene and Organic Molecules" Nanomaterials 9, no. 8: 1136. https://doi.org/10.3390/nano9081136