Additive-Free Rice Starch-Assisted Synthesis of Spherical Nanostructured Hematite for Degradation of Dye Contaminant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spherical Nanostructured Hematite (Sp-HNP)
2.2. Characterization
2.3. Catalytic Activity
3. Results and Discussion
3.1. Characterization of Spherical Hematite (Sp-HNP)
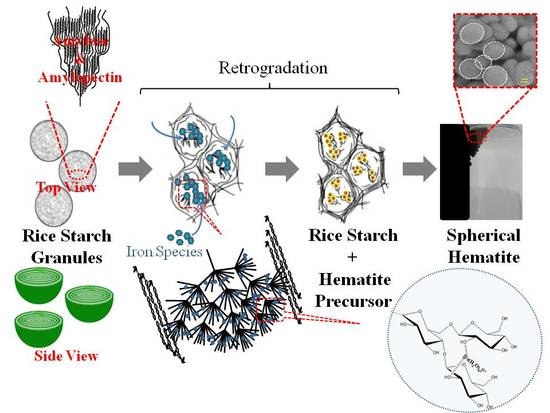
3.2. Plausible Formation of Spherical Hematite (Sp-HNP)
3.3. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karagoz, B.; Yeow, J.; Esser, L.; Prakash, S.M.; Kuchel, R.P.; Davis, T.P.; Boyer, C. An Efficient and Highly Versatile Synthetic Route to Prepare Iron Oxide Nanoparticles/Nanocomposites with Tunable Morphologies. Langmuir 2014, 30, 10493–10502. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cushing, S.K.; Zheng, P.; Meng, F.; Chu, D.; Wu, N. Plasmon-Induced Photonic and Energy-Transfer Enhancement of Solar Water Splitting by a Hematite Nanorod Array. Nat. Commun. 2013, 4, 2651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, H.; Zhuang, J.; Wang, X. Morphology-Controlled Synthesis of Hematite Nanocrystals and Their Facet Effects on Gas-Sensing Properties. Inorg. Chem. 2011, 50, 10143–10151. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.A.; Wang, G.; Ling, Y.; Li, Y.; Zhang, J.Z. Nanostructured Hematite: Synthesis, Characterization, Charge Carrier Dynamics and Photoelectrochemical Properties. Energy Environ. Sci. 2012, 5, 6682–6702. [Google Scholar] [CrossRef]
- Ali, A.; Hira Zafar, M.Z.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.E.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Suzuki, H.; Takahashi, K.; Matijević, E. Reversible Ordered Agglomeration of Hematite Particles due to Weak Magnetic Interactions. J. Colloid Interface Sci. 1986, 113, 76–80. [Google Scholar] [CrossRef]
- Das, P.; Mondal, B.; Mukherjee, K. Facile Synthesis of Pseudo-Peanut Shaped Hematite Iron Oxide Nano-Particles and Their Promising Ethanol and Formaldehyde Sensing Characteristics. RSC Adv. 2014, 4, 31879–31886. [Google Scholar] [CrossRef]
- Patra, A.K.; Kundu, S.K.; Bhaumik, A.; Kim, D. Morphology Evolution of Single-Crystalline Hematite Nanocrystals: Magnetically Recoverable Nanocatalysts for Enhanced Facet-Driven Photoredox Activity. Nanoscale 2016, 8, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, D.; Panjan, M.; Kopanja, L.; Tadić, M. Hydrothermal Synthesis, Morphology, Magnetic Properties and Self-Assembly of Hierarchical α-Fe2O3 (Hematite) Mushroom-, Cube-and Sphere-like Superstructures. Appl. Surf. Sci. 2018, 457, 427–438. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Meng, D.; Qin, X.; Diao, G. Hydrothermal Synthesis of Hematite Nanoparticles and Their Electrochemical Properties. J. Phys. Chem. C 2012, 116, 16276–16285. [Google Scholar] [CrossRef]
- Doermbach, K.; Pich, A. Facile Synthesis of Dumbbell-Shaped Multi-Compartment Nanoparticles. Nanoscale 2015, 7, 9169–9173. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Schultz, E.A.; Sun, T.; Meade, T.; Dravid, V.P. Synthesis of Amine-Stabilized Aqueous Colloidal Iron Oxide Nanoparticles. Cryst. Growth Des. 2007, 7, 471–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.; Sun, D.; Xu, Y.; Liu, P.; Zhang, G.; Sun, Y.; Gao, D. Hematite Nanoplates: Controllable Synthesis, Gas Sensing, Photocatalytic and Magnetic Properties. J. Colloid Interface Sci. 2016, 462, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Asenath-Smith, E.; Estroff, L.A. Role of Akaganeite (β-FeOOH) in the Growth of Hematite (α-Fe2O3) in an Inorganic Silica Hydrogel. Cryst. Growth Des. 2015, 15, 3388–3398. [Google Scholar] [CrossRef]
- Gnanaprakash, G.; Mahadevan, S.; Jayakumar, T.; Kalyanasundaram, P.; Philip, J.; Raj, B. Effect of Initial pH and Temperature of Iron Salt Solutions on Formation of Magnetite Nanoparticles. Mater. Chem. Phys. 2007, 103, 168–175. [Google Scholar] [CrossRef]
- Hao, T.; Yang, C.; Rao, X.; Wang, J.; Niu, C.; Su, X. Facile Additive-Free Synthesis of Iron Oxide Nanoparticles for Efficient Adsorptive Removal of Congo Red and Cr (VI). Appl. Surf. Sci. 2014, 292, 174–180. [Google Scholar] [CrossRef]
- Janardhanan, S.K.; Ramasamy, I.; Nair, B.U. Synthesis of Iron Oxide Nanoparticles Using Chitosan and Starch Templates. Transit. Metal Chem. 2008, 33, 127–131. [Google Scholar] [CrossRef]
- Kumar, S.V.; Bafana, A.P.; Pawar, P.; Rahman, A.; Dahoumane, S.A.; Jeffryes, C.S. High Conversion Synthesis of <10 nm Starch-Stabilized Silver Nanoparticles Using Microwave Technology. Sci. Rep. 2018, 8, 5106. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, H.U.; Oh, S.Y.; Jang, S.C.; Lee, S.C.; Huh, Y.S. Self-Assembled/Oval-Shaped Iron Oxide Nanoparticles for Efficient Photo-Fenton Reaction at Neutral pH. J. Nanosci. Nanotechnol. 2017, 17, 7651–7655. [Google Scholar] [CrossRef]
- Park, C.; Jung, J.; Lee, C.W.; Cho, J. Synthesis of Mesoporous α-Fe2O3 Nanoparticles by Non-Ionic Soft Template and Their Applications to Heavy Oil Upgrading. Sci. Rep. 2016, 6, 39136. [Google Scholar] [CrossRef] [PubMed]
- Matmin, J.; Affendi, I.; Endud, S. Direct-Continuous Preparation of Nanostructured Titania-Silica Using Surfactant-Free Non-Scaffold Rice Starch Template. Nanomaterials 2018, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Darracq, M.A.; Clark, R.F. A Review of Methylene Blue Treatment for Cardiovascular Collapse. J. Emerg. Med. 2014, 46, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.M.; Fowler, P.A.; Sayers, C.; Williams, P.A. The Chemical Modification of a Range of Starches under Aqueous Reaction Conditions. Carbohydr. Polym. 2004, 55, 283–289. [Google Scholar] [CrossRef]
- Goheen, S.M.; Wool, R.P. Degradation of Polyethylene–Starch Blends in Soil. J. Appl. Polym. Sci. 1991, 42, 2691–2701. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Wu, X. Depressing Iron Mineral by Metallic-Starch Complex (MSC) in Reverse Flotation and Its Mechanism. Minerals 2018, 8, 85. [Google Scholar] [CrossRef]
- Justus, J.S.; Roy, S.D.D.; Raj, A.M.E. Synthesis and Characterization of Hematite Nanopowders. Mater. Res. Express 2016, 3, 105037. [Google Scholar] [CrossRef]
- Wang, T.L.; Bogracheva, T.Y.; Hedley, C.L. Starch: As simple as A, B, C? J. Exp. Bot. 1998, 49, 481–502. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch Granules: Structure and Biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Chen, J.; Liu, J.; Wu, H.; Zhan, H.; Liang, C.; Wu, M. Continuous Shape-and Spectroscopy-Tuning of Hematite Nanocrystals. Inorg. Chem. 2010, 49, 8411–8420. [Google Scholar] [CrossRef] [PubMed]
- Catti, M.; Valerio, G.; Dovesi, R. Theoretical Study of Electronic, Magnetic, and Structural Properties of α-Fe2O3 (Hematite). Phys. Rev. B 1995, 51, 7441. [Google Scholar] [CrossRef]
- Liu, C.; Ma, J.; Liu, Y. Formation Mechanism and Magnetic Properties of Three Different Hematite Nanostructures Synthesized by One-Step Hydrothermal Procedure. Sci. China Chem. 2011, 54, 1607. [Google Scholar] [CrossRef]
- Chikate, R.C.; Jun, K.W.; Rode, C.V. Nonaqueous Synthesis and Characterization of Capped α-Fe2O3 Nanoparticles from Iron (III) Hydroxy-Oleate Precursor. Polyhedron 2008, 27, 933–938. [Google Scholar] [CrossRef]
- Zaumseil, P. X-ray Measurement of the Tetragonal Distortion of the Oxide Buffer Layer in Ge/Pr2O3/Si (1 1 1) Heteroepitaxial Structures. J. Phys. D Appl. Phys. 2008, 41, 135308. [Google Scholar] [CrossRef]
- Sayed, F.N.; Polshettiwar, V. Facile and Sustainable Synthesis of Shaped Iron Oxide Nanoparticles: Effect of Iron Precursor Salts on the Shapes of Iron Oxides. Sci. Rep. 2015, 5, 9733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basavegowda, N.; Mishra, K.; Lee, Y.R. Synthesis, Characterization, and Catalytic Applications of Hematite (α-Fe2O3) Nanoparticles as Reusable Nanocatalyst. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025017. [Google Scholar] [CrossRef]
- Dutta, R.K.; Sahu, S. Development of Oxaliplatin Encapsulated in Magnetic Nanocarriers of Pectin as a Potential Targeted Drug Delivery for Cancer Therapy. Res. Pharm. Sci. 2012, 2, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mohammadikish, M. Hydrothermal Synthesis, Characterization and Optical Properties of Ellipsoid Shape α-Fe2O3 Nanocrystals. Ceram. Int. 2014, 40, 1351–1358. [Google Scholar] [CrossRef]
- Ahmmad, B.; Leonard, K.; Islam, M.S.; Kurawaki, J.; Muruganandham, M.; Ohkubo, T.; Kuroda, Y. Green Synthesis of Mesoporous Hematite (α-Fe2O3) Nanoparticles and Their Photocatalytic Activity. Adv. Powder Technol. 2013, 24, 160–167. [Google Scholar] [CrossRef]
- Wu, C.; Yin, P.; Zhu, X.; OuYang, C.; Xie, Y. Synthesis of Hematite (α-Fe2O3) Nanorods: Diameter-Size and Shape Effects on Their Applications in Magnetism, Lithium Ion Battery and Gas Sensors. J. Phys. Chem. B 2006, 110, 17806–17812. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Tao, D.; Zhang, L. Cellulose Scaffold: A Green Template for the Controlling Synthesis of Magnetic Inorganic Nanoparticles. Powder Technol. 2012, 217, 502–509. [Google Scholar] [CrossRef]
- Novoselova, L.Y. Hematite Nanopowder Obtained from Waste: Iron-Removal Sludge. Powder Technol. 2016, 287, 364–372. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, L.; Wang, W.; Zhang, L.; Wu, Q.; Li, J.; Guo, L. Hierarchial Mesoporous Hematite with “Electron-Transport Channels” and Its Improved Performances in Photocatalysis and Lithium Ion Batteries. J. Phys. Chem. C 2011, 115, 7126–7133. [Google Scholar] [CrossRef]
- He, Y.P.; Miao, Y.M.; Li, C.R.; Wang, S.Q.; Cao, L.; Xie, S.S.; Yang, G.Z.; Zou, B.S.; Burda, C. Size and Structure Effect on Optical Transitions of Iron Oxide Nanocrystals. Phys. Rev. B 2005, 71, 125411. [Google Scholar] [CrossRef]
- Sherman, D.M. The Electronic Structures of Fe3+ Coordination Sites in Iron Oxides: Applications to Spectra, Bonding, and Magnetism. Phys. Chem. Miner. 1985, 12, 161–175. [Google Scholar] [CrossRef]
- Grinberg, I.; West, D.V.; Torres, M.; Gou, G.; Stein, D.M.; Wu, L.; Chen, G.; Gallo, E.M.; Akbashev, A.R.; Davies, P.K.; et al. Perovskite Oxides for Visible-Light-Absorbing Ferroelectric and Photovoltaic Materials. Nature 2013, 503, 509. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Surya, R.; Filser, S.; Wimmer, A.; Weigl, F.; Fraga-García, P.; Berensmeier, S. Formation of Iron Oxide Nanoparticles for the Photooxidation of Water: Alteration of Finite Size Effects from Ferrihydrite to Hematite. Sci. Rep. 2017, 7, 12609. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, W.; Tomasik, P. Complexes of Amylose and Amylopectins with Multivalent Metal Salts. J. Inorg. Biochem. 2004, 98, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef] [Green Version]
- Buazar, F.; Bavi, M.; Kroushawi, F.; Halvani, M.; Khaledi-Nasab, A.; Hossieni, S.A. Potato Extract as Reducing Agent and Stabiliser in a Facile Green One-Step Synthesis of ZnO Nanoparticles. J. Exp. Nanosci. 2016, 11, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A Novel One-Pot ‘Green’ Synthesis of Stable Silver Nanoparticles Using Soluble Starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Chairam, S.; Poolperm, C.; Somsook, E. Starch Vermicelli Template-Assisted Synthesis of Size/Shape-Controlled Nanoparticles. Carbohydr. Polym. 2009, 75, 694–704. [Google Scholar] [CrossRef]
- Galli, M.; Guerrini, A.; Cauteruccio, S.; Thakare, P.; Dova, D.; Orsini, F.; Arosio, P.; Carrara, C.; Sangregorio, C.; Lascialfari, A.; et al. Superparamagnetic Iron Oxide Nanoparticles Functionalized by Peptide Nucleic Acids. RSC Adv. 2017, 7, 15500–15512. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.; Roy, V.A. Recent Progress In Magnetic Iron Oxide–Semiconductor Composite Nanomaterials As Promising Photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Redl, F.X.; Black, C.T.; Papaefthymiou, G.C.; Sandstrom, R.L.; Yin, M.; Zeng, H.; Murray, C.B.; O’Brien, S.P. Magnetic, Electronic, and Structural Characterization of Nonstoichiometric Iron Oxides at the Nanoscale. J. Am. Chem. Soc. 2004, 126, 14583–14599. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barick, K.C.; Bahadur, D. Fe3O4 Embedded ZnO Nanocomposites for the Removal of Toxic Metal Ions, Organic Dyes and Bacterial Pathogens. J. Mater. Chem. A 2013, 1, 3325–3333. [Google Scholar] [CrossRef]
- Mansour, A.M. Photocatalytic Degradation of Methylene Blue with Hematite Nanoparticles Synthesized by Thermal Decomposition of Fluoroquinolones Oxalato–Iron (III) Complexes. RSC Adv. 2015, 5, 62052–62061. [Google Scholar] [CrossRef]
- Mardani, H.R.; Forouzani, M.; Ziari, M.; Biparva, P. Visible Light Photo-Degradation of Methylene Blue over Fe or Cu Promoted ZnO Nanoparticles. Spectrochim. Acta Part A 2015, 141, 27–33. [Google Scholar] [CrossRef] [PubMed]










| Elements | Atomic Percentage |
|---|---|
| Platinum (Pt) | 5.47 |
| Oxygen (O) | 58.27 |
| Iron (Fe) | 35.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matmin, J.; Affendi, I.; Ibrahim, S.I.; Endud, S. Additive-Free Rice Starch-Assisted Synthesis of Spherical Nanostructured Hematite for Degradation of Dye Contaminant. Nanomaterials 2018, 8, 702. https://doi.org/10.3390/nano8090702
Matmin J, Affendi I, Ibrahim SI, Endud S. Additive-Free Rice Starch-Assisted Synthesis of Spherical Nanostructured Hematite for Degradation of Dye Contaminant. Nanomaterials. 2018; 8(9):702. https://doi.org/10.3390/nano8090702
Chicago/Turabian StyleMatmin, Juan, Irwan Affendi, Salizatul Ilyana Ibrahim, and Salasiah Endud. 2018. "Additive-Free Rice Starch-Assisted Synthesis of Spherical Nanostructured Hematite for Degradation of Dye Contaminant" Nanomaterials 8, no. 9: 702. https://doi.org/10.3390/nano8090702