Chelator-Free Copper-64-Incorporated Iron Oxide Nanoparticles for PET/MR Imaging: Improved Radiocopper Stability and Cell Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Copper-64
2.3. nat,64Cu(acac)2 Synthesis
2.4. Core-Shell Structures of IO and 64Cu-IO
2.5. Characterization
2.6. Magnetism and Relaxivity
2.7. Cytotoxicity Effects
2.8. Simulated Body Fluid (SBF) Tests
2.9. PET Experiments
3. Results and Discussion
3.1. Synthesis and Characterization of 64Cu-IO@SiO2 NPs
3.2. Magnetic Properties of NPs
3.3. MR Relaxivities of IO and 64Cu-IO NPs at 4.7 T and 9.4 T
3.4. Biocompatibility of Cu-IO@SiO2 In Vitro
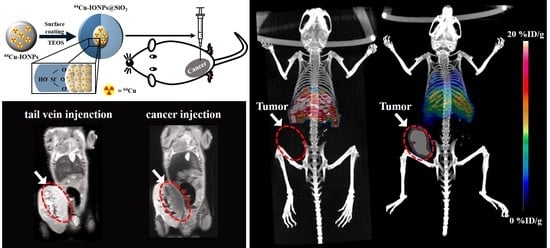
3.5. PET/MR Images of 64Cu-IO@SiO2 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.C.; Yu, M.K.; An, G.I.; Park, S.I.; Oh, J.; Kim, H.J.; Kim, J.H.; Wang, E.K.; Hong, I.H.; Ha, Y.S.; et al. Facile Preparation of a Hybrid Nanoprobe for Triple-Modality Optical/PET/MR Imaging. Small 2010, 6, 2863–2868. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.L.; Zhang, Y.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 Core/Shell Nanoparticles: The Silica Coating Regulations with a Single Core for Different Core Sizes and Shell Thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Chen, F.; Ellison, P.A.; Lewis, C.M.; Hong, H.; Zhang, Y.; Shi, S.; Hernandez, R.; Meyerand, M.E.; Barnhart, T.E.; Cai, W. Chelator-free synthesis of a dual-modality PET/MRI agent. Angew. Chem. Int. Ed. 2013, 52, 13319–13323. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Valdovinos, H.F.; Chen, F.; Lewis, C.M.; Ellison, P.A.; Luo, H.; Meyerand, M.E.; Nickles, R.J.; Cai, W. Intrinsically Germanium-69-labeled iron oxide nanoparticles: Synthesis and in-vivo dual-modality PET/MR Imaging. Adv. Mater. 2014, 26, 5119–5123. [Google Scholar] [CrossRef]
- Zhao, Y.; Sultan, D.; Detering, L.; Cho, S.; Sun, G.; Pierce, R.; Wooley, K.L.; Liu, Y. Copper-64-alloyed gold nanoparticles for cancer imaging: Improved radiolabel stability and diagnostic accuracy. Angew. Chem. Int. Ed. 2014, 53, 156–159. [Google Scholar] [CrossRef]
- Forte, E.; Fiorenza, D.; Torino, E.; di Polidoro, A.C.; Cavaliere, C.; Netti, P.A. Marco Salvatore and Marco Aiello, Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2019, 9, 89. [Google Scholar] [CrossRef]
- Wong, R.M.; Gilbert, D.; Liu, K.; Louie, A.Y. Rapid Size-Controlled Synthesis of Dextran-Coated, 64Cu-Doped Iron Oxide Nanoparticles. ACS Nano 2012, 6, 3461–3467. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Ruiz-Cabello, J.; Saiz-Alia, M.; del Rosario, G.; Caja, S.; Montoya, M.; de Manuel, L.F.; Morales, M.P.; Gurierrez, L.; Galiana, B.; et al. Fast synthesis and bioconjugation of 68Ga core-doped extremely small iron oxide nanoparticles for PET/MR imaging. Contrast Media Mol. Imaging 2015, 11, 203–210. [Google Scholar] [CrossRef]
- Chen, F.; Goel, S.; Valdovinos, H.F.; Luo, H.; Hernandez, R.; Barnhart, T.E.; Cai, W. In vivo integrity and biological fate of chelator-free zirconium-89-labeled mesoporous silica nanoparticles. ACS Nano 2015, 9, 7950–7959. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Ma, W.; Bakr, A.; Rahaman, M.S. pH-sensitive and mangetically separable Fe/Cu bimetallic nanoparticles supported by graphene oxide (GO) for high- efficiency removal of tetracyclines. J. Colloid Interface Sci. 2019, 534, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Glaus, C.; Rossin, R.; Welch, M.J.; Bao, G. In Vivo Evaluation of 64Cu-Labled Magnetic Nanoparticles as a Dual-Modality PET/MR Imaging Agent. Bioconj. Chem. 2010, 21, 715–722. [Google Scholar] [CrossRef]
- Thomas, G.; Boudon, J.; Maurizi, L.; Moreau, M.; Walker, P.; Severin, I.; Oudot, A.; Goze, C.; Poty, S.; Vrigneaud, J.-M.; et al. Innovative Magnetic Nanoparticles for PET/MRI Bimodal Imaging. ACS Omega 2019, 4, 2637–2648. [Google Scholar] [CrossRef]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.S.; Silva, R.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Kilic, G.; Costa, C.; Fernández-Bertólez, N.; Pásaro, E.; ao Paulo Teixeira, J.; Laf-fon, B.; Valdiglesias, V. In vitro toxicity evaluation of silica-coated iron oxide nanoparticles in human SHSY5Y neuronal cells. Toxicol. Res. 2016, 5, 235–247. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Matteis, V.D.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanas-siou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef]
- Wang, Y.; Muramatsu, A.; Sugimoto, T. FTIR analysis of well-defined α-Fe203 particles. Colloid Sand Surf. A Physicochem. Eng. Asp. 1998, 134, 281–297. [Google Scholar] [CrossRef]
- Renuga, D.; Jeyasundari, J.; Athithan, A.S.S.; Jacob, Y.B.A. Synthesis and characterization of copper oxide nanoparticles using Brassica oleracea var. italic extract for its antifungal application. Mater. Res. Express 2020, 7, 45007. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Meng, X.; Li, Y.; Zhao, J. Structural evolution and characteristics of the phase transformations between α- Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmospheres. CrystEngComm 2013, 15, 8166–8172. [Google Scholar] [CrossRef]
- Park, J.K.; Jang, H.M.; Cho, W.-J.; Kim, C.; Suk, J.; Kim, D.S.; Lee, J.S. Enhanced anomalous magnetization in carbonyl iron by Ni+ ion beam irradiation. Sci. Rep. 2021, 11, 20118. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Liu, W. Photocatalytic activity of ZnO/CuO/ZnFe2O4 nanocomposite as a photofenton-like catalyst. Catalysts 2018, 8, 557. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, M.; Upadhyay, C. Signatures of consolidated superparamagnetic and spin-glass behavior in magnetitie-silver core-shell nanoparticles. Nanoscale 2018, 10, 22583–22592. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, G.M.; Alsmadi, A.M.; Alna’washi, G.A.; Salameh, B.; Shatnawi, M.; Al-nemrat, S.; Albiss, B.A.; Bsoul, I. Coexistence of superparamagnetism and spin-glass like behavior in zinc-substituted cobalt ferrite nanoparticles. Appl. Phys. A 2020, 126, 512. [Google Scholar] [CrossRef]
- Greculeasa, S.G.; Palade, P.; Schinteie, G.; Leca, A.; Dumitrache, F.; Lungu, I.; Pro-dan, G.; Kuncser, A.; Kuncser, V. Tuning structural and magnetic properties of Fe oxide nanoparticles by specific hydrogenation treatments. Sci. Rep. 2020, 10, 17174. [Google Scholar] [CrossRef]
- Norek, M.; Kampert, E.; Zeitler, U.; Peters, J.A. Tuning of the Size of Dy2O3 Nanoparticles for Optimal Performance as an MRI Contrast Agent. J. Am. Chem. Soc. 2008, 130, 5335–5340. [Google Scholar] [CrossRef]
- Norek, M.; Peters, J.A. MRI contrast agents based on dysprosium or holmium. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 64–82. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.C.; Nah, H.; Woo, S.; Oh, J.; Kim, K.M.; Cheon, G.J.; Chang, Y.; Yoo, J.; Cheon, J. A Hybrid Nanoparticle Probe for Dual−Modality Positron Emission Tomography and Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2008, 33, 6259–6262. [Google Scholar] [CrossRef]
- Madru, R.; Budassi, M.; Benveniste, H.; Lee, H.; Smith, S.D.; Schlyer, D.J.; Vaska, P.; Knutsson, L.; Strand, S.-E. Simultaneous preclinical positron emission tomography-magnetic resonance imaging study of lymphatic drainage of chelator-free 64Cu-labeled nanoparticles. Cancer Biother. Radiopharm. 2018, 33, 213–220. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.; Huang, M.; Lu, W.; Song, S.; Melancon, M.P.; Tian, M.; Liang, D.; Li, C. A chelator-free multifunctional [64Cu]CuS nanoparticles platform for simultaneous micro-PET/CT imaging and photothermal ablation therpy. J. Am. Chem. Soc. 2010, 132, 15351–15358. [Google Scholar] [CrossRef]
- De Kruijff, R.M.; Raavé, R.; Kip, A.; Molkenboer-Kuenen, J.; Roobol, S.J.; Essers, J.; Heskamp, S.; Denkova, A.G. Elucidating the influence of tumor presence on the poly-mersome circulation time in mice. Pharmaceutics 2019, 11, 241. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Li, Y.; Li, Y.; Zhou, X.; Huang, P.; Sun, Z. Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint. PLoS ONE 2013, 8, e62087. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zang, F.; Wu, H.; Li, J.; Xie, J.; Ma, M.; Gu, N.; Zhang, Y. Using PEGylated magnetic nanoparticles to describe EPR effect in tumor for predicting therapeutic efficacy of micelle drugs. Nanoscale 2018, 10, 1788–1797. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T1–T2 switchable magnetic resonance imaging contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef]
- Geppert, M.; Himly, M. Iron oxide nanoparticles in bioimaging-an immune perspective. Front. Immunol. 2021, 12, 688927. [Google Scholar] [CrossRef]
- Holder, A.L.; Vejerano, E.P.; Zhou, X.; Marr, L.C. Nanomaterial disposal by incineration. Environ. Sci. Processes Impacts 2013, 15, 1652. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.M.; Jung, M.H.; Lee, J.S.; Lee, J.S.; Lim, I.-C.; Im, H.; Kim, S.W.; Kang, S.-A.; Cho, W.-J.; Park, J.K. Chelator-Free Copper-64-Incorporated Iron Oxide Nanoparticles for PET/MR Imaging: Improved Radiocopper Stability and Cell Viability. Nanomaterials 2022, 12, 2791. https://doi.org/10.3390/nano12162791
Jang HM, Jung MH, Lee JS, Lee JS, Lim I-C, Im H, Kim SW, Kang S-A, Cho W-J, Park JK. Chelator-Free Copper-64-Incorporated Iron Oxide Nanoparticles for PET/MR Imaging: Improved Radiocopper Stability and Cell Viability. Nanomaterials. 2022; 12(16):2791. https://doi.org/10.3390/nano12162791
Chicago/Turabian StyleJang, Hye Min, Myung Hwan Jung, Jae Sang Lee, Jun Sig Lee, In-Cheol Lim, Hyunsik Im, Sang Wook Kim, Sung-A Kang, Won-Je Cho, and Jun Kue Park. 2022. "Chelator-Free Copper-64-Incorporated Iron Oxide Nanoparticles for PET/MR Imaging: Improved Radiocopper Stability and Cell Viability" Nanomaterials 12, no. 16: 2791. https://doi.org/10.3390/nano12162791