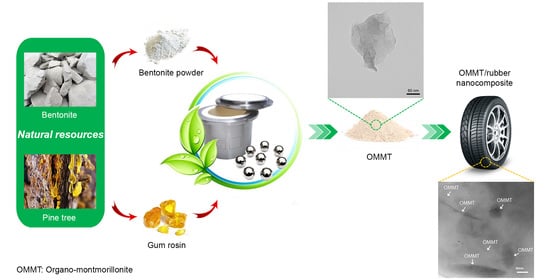
Mechanochemical Synthesis of Rosin-Modified Montmorillonite: A Breakthrough Approach to the Next Generation of OMMT/Rubber Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. OMMT Synthesis
2.3. Preparation of OMMT/Rubber Nanocomposites
2.4. Materials Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, T.; Damtie, M.M.; Hosseinzadeh, A.; Wei, W.; Jin, J.; Phong Vo, H.N.; Ye, J.S.; Liu, Y.; Wang, X.F.; Yu, Z.M.; et al. Bentonite-supported nano zero-valent iron composite as a green catalyst for bisphenol A degradation: Preparation, performance, and mechanism of action. J. Environ. Manag. 2020, 260, 110105. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabi, H.H.; Ali, H.R.; Zahran, F.; Betiha, M.A. Functionalized magnetic bentonite-iron oxide nanocomposite and its application to decrease scale formation in tubing of oil/gas production. Appl. Surf. Sci. Adv. 2021, 4, 100058. [Google Scholar] [CrossRef]
- Shakeel, A.; Kirichek, A.; Chassagne, C. Rheology and yielding transitions in mixed kaolinite/bentonite suspensions. Appl. Clay Sci. 2021, 211, 106206. [Google Scholar] [CrossRef]
- Ranđelović, M.S.; Purenović, M.M.; Matović, B.Z.; Zarubica, A.R.; Momčilović, M.Z.; Purenović, J.M. Structural, textural and adsorption characteristics of bentonite-based composite. Microporous Mesoporous Mater. 2014, 195, 67–74. [Google Scholar] [CrossRef]
- Xiao, F.; Yan, B.Q.; Zou, X.Y.; Cao, X.Q.; Dong, L.; Lyu, X.J.; Li, L.; Qiu, J.; Chen, P.; Hu, S.G.; et al. Study on ionic liquid modified montmorillonite and molecular dynamics simulation. Colloids Surf. Physicochem. Eng. Asp. 2020, 587, 124311. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef] [Green Version]
- De Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Pandey, J.K.; Raghunatha Reddy, K.; Pratheep Kumar, A.; Singh, R.P. An overview on the degradability of polymer nanocomposites. Polym. Degrad. Stab. 2005, 88, 234–250. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Guégan, R. Organoclay applications and limits in the environment. Comptes Rendus Chim. 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Ltifi, I.; Ayari, F.; Chehimi, D.B.H.; Ayadi, M.T. Physicochemical characteristics of organophilic clays prepared using two organo-modifiers: Alkylammonium cation arrangement models. Appl. Water Sci. 2018, 8, 91. [Google Scholar] [CrossRef]
- Dongmo, L.M.; Jiokeng, S.L.Z.; Pecheu, C.N.; Walcarius, A.; Tonle, I.K. Amino-grafting of montmorillonite improved by acid activation and application to the electroanalysis of catechol. Appl. Clay Sci. 2020, 191, 105602. [Google Scholar] [CrossRef]
- Paul, B.; Martens, W.N.; Frost, R.L. Organosilane grafted acid-activated beidellite clay for the removal of non-ionic alachlor and anionic imazaquin. Appl. Surf. Sci. 2011, 257, 5552–5558. [Google Scholar] [CrossRef]
- Hayakawa, T.; Minase, M.; Fujita, K.I.; Ogawa, M. Green synthesis of organophilic clays; solid-state reaction of acidic clay with organoamine. Ind. Eng. Chem. Res. 2016, 55, 6325–6330. [Google Scholar] [CrossRef]
- Cardona, Y.; Korili, S.A.; Gil, A. Understanding the formation of Al13 and Al30 polycations to the development of microporous materials based on Al13-and Al30-PILC montmorillonites: A review. Appl. Clay Sci. 2021, 203, 105996. [Google Scholar] [CrossRef]
- Fang, L.; Wang, L.; Zhou, T.; Liu, L.; Zhou, J.; Li, M. Preparation and characterization of Fe,Co,Si-pillared montmorillonites with aminosilanes as silicon pillars precursor. Appl. Clay Sci. 2017, 141, 88–94. [Google Scholar] [CrossRef]
- Zha, W.; Han, C.D.; Han, S.H.; Lee, D.H.; Kim, J.K.; Guo, M.; Rinaldi, P.L. Ion–dipole interactions in the dispersion of organoclay nanocomposites based on polystyrene-block-poly(2-vinylpyridine) copolymer. Polymer 2009, 50, 2411–2423. [Google Scholar] [CrossRef]
- Murtaza, M.; Ahmad, H.M.; Kamal, M.S.; Hussain, S.M.; Mahmoud, M.; Patil, S. Evaluation of clay hydration and swelling inhibition using quaternary ammonium dicationic surfactant with phenyl linker. Molecules 2020, 25, 4333. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Yu, Z.G.; Zhu, M.Y.; Shen, L.Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- Yapar, S. Physicochemical study of microwave-synthesized organoclays. Colloids Surf. Physicochem. Eng. Asp. 2009, 345, 75–81. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Cai, W. Characteristics of organobentonite prepared by microwave as a sorbent to organic contaminants in water. Colloids Surf. Physicochem. Eng. Asp. 2006, 281, 177–183. [Google Scholar] [CrossRef]
- Baláž, M.; Achimovičová, M.; Baláž, P.; Dutková, E.; Fabián, M.; Kováčová, M.; Lukáčová Bujňáková, Z.; Tóthová, E. Mechanochemistry as a versatile and scalable tool for nanomaterials synthesis: Recent achievements in Košice, Slovakia. Curr. Opin. Green Sustain. Chem. 2020, 24, 7–13. [Google Scholar] [CrossRef]
- Dinda, S.; Bhagavatam, A.; Alrehaili, H.; Dinda, G.P. Mechanochemical synthesis of nanocrystalline hydroxyapatite from Ca(H2PO4)2.H2O, CaO, Ca(OH)2, and P2O5 mixtures. Nanomaterials 2020, 10, 2232. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q. Application of mechanochemical synthesis of advanced materials. J. Adv. Ceram. 2012, 1, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- Do, J.L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, R.T.; Boulatov, R. The many flavours of mechanochemistry and its plausible conceptual underpinnings. Nat. Rev. Chem. 2021, 5, 148–167. [Google Scholar] [CrossRef]
- Tan, D.; García, F. Main group mechanochemistry: From curiosity to established protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Handa, T.; Kuroda, K.; Kato, C. Formation of organoammonium-montmorillonites by solid-solid reactions. Chem. Lett. 1990, 19, 71–74. [Google Scholar] [CrossRef]
- Ogawa, M.; Kato, K.; Kuroda, K.; Kato, C. Preparation of montmorillonite-alkylamine intercalation compounds by solid-solid reactions. Clay Sci. 1990, 8, 31–36. [Google Scholar] [CrossRef]
- Guégan, R. Intercalation of a nonionic surfactant (C10E3) bilayer into a Na-montmorillonite clay. Langmuir 2010, 26, 19175–19180. [Google Scholar] [CrossRef] [Green Version]
- Khaorapapong, N.; Ogawa, M. Solid-state intercalation of organic and inorganic substances in smectites. Clay Sci. 2011, 15, 147–159. [Google Scholar] [CrossRef]
- Petra, L.; Billik, P.; Komadel, P. Preparation and characterization of hybrid materials consisting of high-energy ground montmorillonite and α-amino acids. Appl. Clay Sci. 2015, 115, 174–178. [Google Scholar] [CrossRef]
- Chen, R.S.; Ahmad, S.; Gan, S. Characterization of recycled thermoplastics-based nanocomposites: Polymer-clay compatibility, blending procedure, processing condition, and clay content effects. Compos. Part B 2017, 131, 91–99. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Aldas, M.; Ferri, J.M.; Lopez-Martinez, J.; Samper, M.D.; Arrieta, M.P. Effect of pine resin derivatives on the structural, thermal, and mechanical properties of Mater-Bi type bioplastic. J. Appl. Polym. Sci. 2020, 137, 48236. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Ferrándiz, S. New materials for 3D-printing based on polycaprolactone with gum rosin and beeswax as additives. Polymers 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Joshi, S.; Malviya, T. Carboxymethyl cellulose-rosin gum hybrid nanoparticles: An efficient drug carrier. Int. J. Biol. Macromol. 2018, 112, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, S.I. Synthesis of acrylic rosin derivatives and application as negative photoresist. Eur. Polym. J. 2002, 38, 387–392. [Google Scholar] [CrossRef]
- Sadhra, S.; Foulds, I.S.; Gray, C.N. Identification of contact allergens in unmodified rosin using a combination of patch testing and analytical chemistry techniques. Br. J. Dermatol. 1996, 134, 662–668. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Liang, J.; Chen, Y.; Pu, X.; Tong, Z. Kinetics of the catalytic isomerization and disproportionation of rosin over carbon-supported palladium. Chem. Eng. J. 2009, 152, 242–250. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Teoh, M.M.; Chung, T.S. Hydrogen separation and purification in membranes of miscible polymer blends with interpenetration networks. Polymer 2008, 49, 1594–1603. [Google Scholar] [CrossRef]
- Lafitte, G.; Espuche, E.; Gérard, J.F. Polyamide 11/poly(hydroxy amino ether) blends: Influence of the blend composition and morphology on the barrier and mechanical properties. Eur. Polym. J. 2011, 47, 1994–2002. [Google Scholar] [CrossRef]
- Ramadan, A.R.; Esawi, A.M.K.; Gawad, A.A. Effect of ball milling on the structure of Na+-montmorillonite and organo-montmorillonite (Cloisite 30B). Appl. Clay Sci. 2010, 47, 196–202. [Google Scholar] [CrossRef]
- Araújo, E.M.; Barbosa, R.; Morais, C.R.S.; Soledade, L.E.B.; Souza, A.G.; Vieira, M.Q. Effects of organoclays on the thermal processing of pe/clay nanocomposites. J. Therm. Anal. Calorim. 2007, 90, 841–848. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L. Chemical and thermal properties of organoclays derived from highly stable bentonite in sulfuric acid. Appl. Clay Sci. 2013, 83–84, 349–356. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.D.; Jasra, R.V. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim. Acta Part A 2006, 64, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kaith, B.S.; Jindal, R.; Sharma, R. Synthesis of a gum rosin alcohol-poly(acrylamide) based adsorbent and its application in removal of malachite green dye from waste water. RSC Adv. 2015, 5, 43092–43104. [Google Scholar] [CrossRef]
- Kaith, B.S.; Jindal, R.; Sharma, R. Study of ionic charge dependent salt resistant swelling behavior and removal of colloidal particles using reduced gum rosin-poly(acrylamide)-based green flocculant. Iran. Polym. J. 2016, 25, 349–362. [Google Scholar] [CrossRef]
- Katti, K.S.; Sikdar, D.; Katti, D.R.; Ghosh, P.; Verma, D. Molecular interactions in intercalated organically modified clay and clay–polycaprolactam nanocomposites: Experiments and modeling. Polymer 2006, 47, 403–414. [Google Scholar] [CrossRef]
- Madejová, J.; Sekeráková, Ľ.; Bizovská, V.; Slaný, M.; Jankovič, Ľ. Near-infrared spectroscopy as an effective tool for monitoring the conformation of alkylammonium surfactants in montmorillonite interlayers. Vib. Spectrosc. 2016, 84, 44–52. [Google Scholar] [CrossRef]
- Kryuchkova, M.; Danilushkina, A.; Lvov, Y.; Fakhrullin, R. Evaluation of toxicity of nanoclays and graphene oxide in vivo: A Paramecium caudatum study. Environ. Sci. Nano 2016, 3, 442–452. [Google Scholar] [CrossRef] [Green Version]
- Donato, D.I.; Lazzara, G.; Milioto, S. Thermogravimetric analysis. J. Therm. Anal. Calorim. 2010, 101, 1085–1091. [Google Scholar] [CrossRef]
- Frances, M. Effect of heat treatment on Pinus pinaster rosin: A study of physico chemical changes and influence on the quality of rosin linseed oil varnish. Ind. Crops Prod. 2020, 155, 2155. [Google Scholar] [CrossRef]
- Jindal, R.; Sharma, R.; Maiti, M.; Kaur, A.; Sharma, P.; Mishra, V.; Jana, A.K. Synthesis and characterization of novel reduced gum rosin-acrylamide copolymer-based nanogel and their investigation for antibacterial activity. Polym. Bull. 2017, 74, 2995–3014. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Niu, M.; Chen, J.; Wen, J.; Bian, H.; Yu, C.; Liang, M.; Ma, L.; Lai, F.; et al. Thermal stability of abietic acid and its oxidation products. Energy Fuels 2019, 33, 11200–11209. [Google Scholar] [CrossRef]
- He, H.; Duchet, J.; Galy, J.; Gérard, J.F. Influence of cationic surfactant removal on the thermal stability of organoclays. J. Colloid Interface Sci. 2006, 295, 202–208. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Ma, Y.; Zhu, J.; Yuan, P.; Qing, Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl. Clay Sci. 2010, 48, 67–72. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Preparation and characterization of new organoclays using natural amino acids and Cloisite Na+. Appl. Clay Sci. 2011, 51, 353–359. [Google Scholar] [CrossRef]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of wyoming montmorillonite surfaces using a cationic surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef]
- Perera, S.J.; Egodage, S.M.; Walpalage, S. Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex. e-Polymers 2020, 20, 144–153. [Google Scholar] [CrossRef]
- Zachariah, A.K.; Geethamma, V.G.; Chandra, A.K.; Mohammed, P.K.; Thomas, S. Rheological behaviour of clay incorporated natural rubber and chlorobutyl rubber nanocomposites. RSC Adv. 2014, 4, 58047–58058. [Google Scholar] [CrossRef]
- Sharma, A.; Payne, S.; Katti, K.S.; Katti, D.R. Evaluating molecular interactions in polycaprolactone-biomineralized hydroxyapatite nanocomposites using steered molecular dynamics. JOM 2015, 67, 733–743. [Google Scholar] [CrossRef]
- Sankaran, K.; Manoharan, P.; Chattopadhyay, S.; Nair, S.; Govindan, U.; Arayambath, S.; Nando, G.B. Effect of hybridization of organoclay with carbon black on the transport, mechanical, and adhesion properties of nanocomposites based on bromobutyl/epoxidized natural rubber blends. RSC Adv. 2016, 6, 33723–33732. [Google Scholar] [CrossRef]
- Hermenegildo, G.; Bischoff, E.; Mauler, R.S.; Giovanela, M.; Carli, L.N.; Crespo, J.S. Development of chlorobutyl rubber/natural rubber nanocomposites with montmorillonite for use in the inner liner of tubeless ride tires. J. Elastomers Plast. 2017, 49, 47–61. [Google Scholar] [CrossRef]
- Carli, L.N.; Roncato, C.R.; Zanchet, A.; Mauler, R.S.; Giovanela, M.; Brandalise, R.N.; Crespo, J.S. Characterization of natural rubber nanocomposites filled with organoclay as a substitute for silica obtained by the conventional two-roll mill method. Appl. Clay Sci. 2011, 52, 56–61. [Google Scholar] [CrossRef]
- Praveen, S.; Chattopadhyay, P.K.; Albert, P.; Dalvi, V.G.; Chakraborty, B.C.; Chattopadhyay, S. Synergistic effect of carbon black and nanoclay fillers in styrene butadiene rubber matrix: Development of dual structure. Compos. Part A 2009, 40, 309–316. [Google Scholar] [CrossRef]
- López-Manchado, M.A.; Arroyo, M.; Herrero, B.; Biagiotti, J. Vulcanization kinetics of natural rubber–organoclay nanocomposites. J. Appl. Polym. Sci. 2003, 89, 1–15. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef] [Green Version]
- Muzny, C.D.; Butler, B.D.; Hanley, H.J.M.; Tsvetkov, F.; Peiffer, D.G. Clay platelet dispersion in a polymer matrix. Mater. Lett. 1996, 28, 379–384. [Google Scholar] [CrossRef]
- Theng, B.K.G. Formation and Properties of Clay-Polymer Complexes; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1979; Volume 4. [Google Scholar]
- Triantafillidis, C.S.; LeBaron, P.C.; Pinnavaia, T.J. Homostructured mixed inorganic−organic ion clays: A new approach to epoxy polymer−exfoliated clay nanocomposites with a reduced organic modifier content. Chem. Mater. 2002, 14, 4088–4095. [Google Scholar] [CrossRef]







| Material Type | Phr (Parts Per Hundred Rubber) |
|---|---|
| Natural and synthetic rubber (including SMR, SBR and chlorobutyl rubber) | 100 |
| Carbon black | 46.59 |
| Aromatic oil | 4.33 |
| Gum rosin | 2.63 |
| Calcium carbonate | 17.4 |
| Stearic acid | 1.13 |
| Magnesium oxide | 0.31 |
| Others (Riwax, Anox, …) | 0.85 |
| Properties | UnModified Inner Liner Composition | 4 Phr OMMT/Rubber Nanocomposite (% Change) | 7 Phr OMMT/Rubber Nanocomposite (% Change) |
|---|---|---|---|
| Tensile strength (MPa) | 10.5 ± 0.3 | 11.1 ± 0.2 (+6.1 ± 0.9) | 10.76 ± 0.2 (+2.5 ± 1.0) |
| Elongation at break (MPa) | 537 ± 11 | 584 ± 10 (+9.0 ± 0.6) | 551 ± 8 (+2.6 ± 0.6) |
| Modulus 300% (MPa) | 5.4 ± 0.1 | 4.9 ± 0.1 (−10.2 ± 0.4) | 5.6 ± 0.1 (+5.6 ± 0.1) |
| Tear resistance (kN/m) | 36.6 ± 1.6 | 42.3 ± 1.2 (+16.0 ± 2.0) | 34.1 ± 1.4 (−6.8 ± 0.2) |
| Hardness (Shore A) | 57 ± 1 | 56 ± 1 (−1.75 ± 0.03) | 56 ± 1 (−1.75 ± 0.03) |
| Mooney viscosity (MU) | 47.6 ± 0.4 | 42.8 ± 0.3 (−10.0 ± 1.4) | 57.1 ± 0.5 (+19.95 ± 0.05) |
| Scorch time (s) | 70 ± 1 | 73 ± 1 (+4.3 ± 0.1) | 72 ± 1 (+2.9 ± 0.1) |
| Air permeation (Barrer) | 2.5 ± 0.1 | 1.26 ± 0.1 (−49.5 ± 2.1) | 1.95 ± 0.03 (−21.7 ± 1.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmaeili, E.; Rounaghi, S.A.; Eckert, J. Mechanochemical Synthesis of Rosin-Modified Montmorillonite: A Breakthrough Approach to the Next Generation of OMMT/Rubber Nanocomposites. Nanomaterials 2021, 11, 1974. https://doi.org/10.3390/nano11081974
Esmaeili E, Rounaghi SA, Eckert J. Mechanochemical Synthesis of Rosin-Modified Montmorillonite: A Breakthrough Approach to the Next Generation of OMMT/Rubber Nanocomposites. Nanomaterials. 2021; 11(8):1974. https://doi.org/10.3390/nano11081974
Chicago/Turabian StyleEsmaeili, Elaheh, Seyyed Amin Rounaghi, and Jürgen Eckert. 2021. "Mechanochemical Synthesis of Rosin-Modified Montmorillonite: A Breakthrough Approach to the Next Generation of OMMT/Rubber Nanocomposites" Nanomaterials 11, no. 8: 1974. https://doi.org/10.3390/nano11081974