Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process
Abstract
:1. Introduction
2. Changes of the Alveolar Process Following Extraction
2.1. Degradation Period
2.2. Healing Period
3. Factors Influencing Alveolar Bone Loss and Regeneration
3.1. Bone Loss Factors
3.2. Bone Regeneration Factors
3.3. Osteoinductive Factors
4. Augmentation Techniques
4.1. Guided Bone Regeneration (GBR)
4.2. Bone Augmentation Methods and Precise Implant Placement
- Class I: bucco-lingual loss of tissue with normal ridge height in an apicocoronal direction;
- Class II: apico-coronal loss of tissue with normal ridge width in a bucco-lingual direction;
- Class III: combination of bucco-lingual and apico-coronal loss of tissue resulting in loss of height and width.
4.3. Membranes in GBR
4.4. Resorbable Membranes
4.5. Autogenous Bone Grafts
4.6. Allografts
4.7. Xenograft
4.8. Bone Substitute Materials and Genetic Engineering
5. Nanomaterials Application in Alveolar Bone Regeneration
5.1. Nanohydroxyapatite (n-HAp)
5.2. Examples of Bone Regeneration Using Nanohydroxyapatite and Stem Cells in Published Studies
5.3. Nanohydroxyapatite Doped with Rare Ions
5.4. Carbon Nanotubes as a Bone Regeneration Scaffold
5.5. 3D-Printed Scaffold Nanomaterials for Bone Application
5.6. Graphene-Based Nanomaterial in Bone Regeneration
5.7. Structural Effects of Nanomaterials on Bone Regeneration
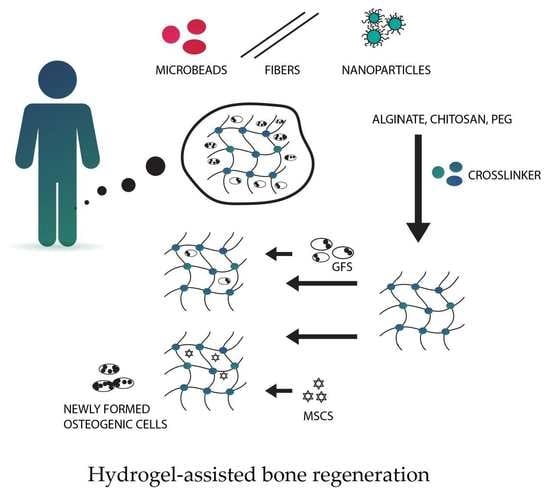
5.8. Hydrogels
5.9. Nanostructured Scaffolds for Bone Tissue Engineering
6. Tissue Engineering-Stem Cell Application in Bone Augmentation
6.1. Mesenchymal Stem Cells Use
6.2. Hematopoietic Stem Cells Use (BMMSCs)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lekholm, U.; Ericsson, I.; Adell, R.; Slots, J. The condition of the soft tissues at tooth and fixture abutments supporting fixed bridges. A microbiological and histological study. J. Clin. Periodontol. 1986, 13, 558–562. [Google Scholar] [CrossRef]
- Sakkas, A.; Ioannis, K.; Winter, K.; Schramm, A.; Wilde, F. Clinical results of autologous bone augmentation harvested from the mandibular ramus prior to implant placement. An analysis of 104 cases. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2016, 5, Doc21. [Google Scholar] [CrossRef]
- Demetriades, N.; Park, J.I.; Laskarides, C. Alternative Bone Expansion Technique for Implant Placement in Atrophic Edentulous Maxilla and Mandible. J. Oral Implantol. 2011, 37, 463–471. [Google Scholar] [CrossRef]
- Basa, S.; Varol, A.; Turker, N. Alternative bone expansion technique for immediate placement of implants in the edentulous posterior mandibular ridge: A clinical report. Int. J. Oral Maxillofac. Implant. 2004, 19, 554–558. [Google Scholar]
- Pistilli, R.; Felice, P.; Piatelli, M.; Nisii, A.; Barausse, C.; Esposito, M. Blocks of autogenous bone versus xenografts for the rehabilitation of atrophic jaws with dental implants: Preliminary data from a pilot randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 153–171. [Google Scholar] [PubMed]
- Goyal, S.; Iyer, S. Bone Manipulation Techniques. Int. J. Clin. Implant. Dent. 2009, 1, 22–23. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Hämmerle, C.H.F. European Workshop on Periodontology Group C Advances in bone augmentation to enable dental implant placement: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 168–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagni, G.; Pellegrini, G.; Giannobile, W.V.; Rasperini, G. Postextraction Alveolar Ridge Preservation: Biological Basis and Treatments. Int. J. Dent. 2012, 2012, 151030. [Google Scholar] [CrossRef] [Green Version]
- Javed, A.; Chen, H.; Ghori, F.Y. Genetic and Transcriptional Control of Bone Formation. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.E.; Repeke, C.E.; Junior, S.D.; Colavite, P.M.; Biguetti, C.C.; Oliveira, R.C.; Assis, G.F.; Taga, R.; Trombone, A.P.F.; Garlet, G.P. Intramembranous bone healing process subsequent to tooth extraction in mice: Micro-computed tomography, histomorphometric and molecular characterization. PLoS ONE 2015, 10, e0128021. [Google Scholar] [CrossRef] [Green Version]
- Bodic, F.; Hamel, L.; Lerouxel, E.; Baslé, M.F.; Chappard, D. Bone loss and teeth. Jt. Bone Spine 2005, 72, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Devlin, H.; Ferguson, M.W. Alveolar ridge resorption and mandibular atrophy. A review of the role of local and systemic factors. Br. Dent. J. 1991, 170, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Mittal, Y.; Jindal, G.; Garg, S. Bone manipulation procedures in dental implants. Indian J. Dent. 2016, 7, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Kanyama, M.; Kuboki, T.; Akiyama, K.; Nawachi, K.; Miyauchi, F.M.; Yatani, H.; Kubota, S.; Nakanishi, T.; Takigawa, M. Connective tissue growth factor expressed in rat alveolar bone regeneration sites after tooth extraction. Arch. Oral Biol. 2003, 48, 723–730. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Infection, Inflammation, and Bone Regeneration: A Paradoxical Relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- DeVlin, H.; Hoyland, J.; Newall, J.F.; Ayad, S. Trabecular Bone Formation in the Healing of the Rodent Molar Tooth Extraction Socket. J. Bone Miner. Res. 1997, 12, 2061–2067. [Google Scholar] [CrossRef]
- Könnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.-D.; Radbruch, A.; Duda, G.N.; et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardaropoli, G.; Araújo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Lang, N.P.; Schweikert, M.T.; de Oliveira, J.A.; Rangel-Garcia, I.; Botticelli, D. Sequential healing of open extraction sockets. An experimental study in monkeys. Clin. Oral Implant. Res. 2014, 25, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar] [CrossRef]
- Reich, K.M.; Huber, C.D.; Lippnig, W.R.; Ulm, C.; Watzek, G.; Tangl, S. Atrophy of the residual alveolar ridge following tooth loss in an historical population. Oral Dis. 2011, 17, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Atwood, D.A. Postextraction changes in the adult mandible as illustrated by microradiographs of midsagittal sections and serial cephalometric roentgenograms. J. Prosthet. Dent. 1963, 13, 810–824. [Google Scholar] [CrossRef]
- Hillmann, G.; Geurtsen, W. Pathohistology of undecalcified primary teeth in vitamin D-resistant rickets: Review and report of two cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 218–224. [Google Scholar] [CrossRef]
- Bender, I.B.; Naidorf, I.J. Dental observations in vitamin D-resistant rickets with special reference to periapical lesions. J. Endod. 1985, 11, 514–520. [Google Scholar] [CrossRef]
- Amler, M.H.; Johnson, P.L.; Salman, I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J. Am. Dent. Assoc. 1960, 61, 32–44. [Google Scholar] [CrossRef]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [Green Version]
- Donaruma, L.G. Definitions in biomaterials, D.F. Williams, Ed., Elsevier, Amsterdam, 1987, 72 pp. J. Polym. Sci. Polym. Lett. Ed. 1988, 26, 414. [Google Scholar] [CrossRef]
- Sykaras, N.; Opperman, L.A. Bone morphogenetic proteins (BMPs): How do they function and what can they offer the clinician? J. Oral Sci. 2003, 45, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Tonelli, P.; Duvina, M.; Barbato, L.; Biondi, E.; Nuti, N.; Brancato, L.; Rose, G.D. Bone regeneration in dentistry. Clin. Cases Miner. Bone Metab. 2011, 8, 24–28. [Google Scholar] [PubMed]
- Aukhil, I.; Simpson, D.M.; Suggs, C.; Pettersson, E. In vivo differentiation of progenitor cells of the periodontal ligament. J. Clin. Periodontol. 1986, 13, 862–868. [Google Scholar] [CrossRef]
- Lewandrowski, K.U.; Bondre, S.P.; Gresser, J.D.; Wise, D.L.; Tomford, W.W.; Trantolo, D.J. Improved osteoconduction of cortical bone grafts by biodegradable foam coating. Biomed. Mater. Eng. 1999, 9, 265–275. [Google Scholar]
- Bengazi, F.; Wennström, J.L.; Lekholm, U. Recession of the soft tissue margin at oral implants. A 2-year longitudinal prospective study. Clin. Oral Implant. Res. 1996, 7, 303–310. [Google Scholar] [CrossRef]
- Mandracchia, V.J.; Nelson, S.C.; Barp, E.A. Current concepts of bone healing. Clin. Podiatr. Med. Surg. 2001, 18, 55–77. [Google Scholar]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [Green Version]
- Kanczler, J.M.; Oreffo, R.O.C. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cell. Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Peng, H.; Usas, A.; Olshanski, A.; Ho, A.M.; Gearhart, B.; Cooper, G.M.; Huard, J. VEGF Improves, Whereas sFlt1 Inhibits, BMP2-Induced Bone Formation and Bone Healing Through Modulation of Angiogenesis. J. Bone Miner. Res. 2005, 20, 2017–2027. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, G.N.; Cochrant, D.L. Factors That Modulate the Effects of Bone Morphogenetic Protein-Induced Periodontal Regeneration: A Critical Review. J. Periodontol. 2002, 73, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Even, J.; Eskander, M.; Kang, J. Bone Morphogenetic Protein in Spine Surgery: Current and Future Uses. J. Am. Acad. Orthop. Surg. 2012, 20, 547–552. [Google Scholar] [CrossRef]
- Hughes, F.J.; Turner, W.; Belibasakis, G.; Martuscelli, G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol. 2000 2006, 41, 48–72. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, K.P.; Elavarasu, S.; Gadagi, J.S. The application of bone morphogenetic proteins to periodontal and peri-implant tissue regeneration: A literature review. J. Pharm. Bioallied Sci. 2012, 4, S427–S430. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Saito, E.; Handa, R.; Honma, Y.; Kawanami, M. Influence of Residual Bone on Recombinant Human Bone Morphogenetic Protein-2–Induced Periodontal Regeneration in Experimental Periodontitis in Dogs. J. Periodontol. 2009, 80, 961–968. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Sampath, T.K.; Rueger, D.C.; Taylor, T.D. Use of bovine osteogenic protein to promote rapid osseointegration of endosseous dental implants. Int. J. Oral Maxillofac. Implant. 1992, 7, 297–301. [Google Scholar]
- Behr, B.; Tang, C.; Germann, G.; Longaker, M.T.; Quarto, N. Locally Applied Vascular Endothelial Growth Factor A Increases the Osteogenic Healing Capacity of Human Adipose-Derived Stem Cells by Promoting Osteogenic and Endothelial Differentiation. Stem Cells 2011, 29, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Kent Leach, J.; Kaigler, D.; Wang, Z.; Krebsbach, P.H.; Mooney, D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.E.; Vranes, D.; Youssef, S.; Stacker, S.A.; Cox, A.J.; Rizkalla, B.; Casley, D.J.; Bach, L.A.; Kelly, D.J.; Gilbert, R.E. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes 1999, 48, 2229–2239. [Google Scholar] [CrossRef]
- Fragiskos, F.D.; Alexandridis, C. Osseointegrated Implants. In Oral Surgery; Springer: Berlin/Heidelberg, Germany, 2018; pp. 337–347. [Google Scholar]
- McAllister, B.S.; Haghighat, K. Bone Augmentation Techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, W.; Becker, B.E.; Handlesman, M.; Celletti, R.; Ochsenbein, C.; Hardwick, R.; Langer, B. Bone formation at dehisced dental implant sites treated with implant augmentation material: A pilot study in dogs. Int. J. Periodontics Restor. Dent. 1990, 10, 92–101. [Google Scholar]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of Bone Defects by Guided Tissue Regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Gher, M.E.; Quintero, G.; Assad, D.; Monaco, E.; Richardson, A.C. Bone Grafting and Guided Bone Regeneration for Immediate Dental Implants in Humans. J. Periodontol. 1994, 65, 881–891. [Google Scholar] [CrossRef]
- Wang, H.-L.; Boyapati, L. “PASS” Principles for Predictable Bone Regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Kostopoulos, L.; Karring, T. Guided bone regeneration in mandibular defects in rats using a bioresorbable polymer. Clin. Oral Implant. Res. 1994, 5, 66–74. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Eskow, R.N.; Zamzok, J. Aesthetics and implant dentistry. Periodontol. 2000 1996, 11, 85–94. [Google Scholar] [CrossRef]
- Alghamdi, H. Methods to Improve Osseointegration of Dental Implants in Low Quality (Type-IV) Bone: An Overview. J. Funct. Biomater. 2018, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Rana, R.; Ramachandra, S.S.; Lahori, M.; Singhal, R.; Jithendra, K.D. Combined soft and hard tissue augmentation for a localized alveolar ridge defect. Contemp. Clin. Dent. 2013, 4, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Hämmerle, C.H.F.; Cochran, D.L.; Jones, A.A.; Görlach, C.; Uebersax, L.; Mathes, S.; Graf-Hausner, U.; Jung, R.E. Soft tissue volume augmentation by the use of collagen-based matrices in the dog mandible—A histological analysis. J. Clin. Periodontol. 2011, 38, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Bagavad Gita, V.; Chandrasekaran, S. Hard and soft tissue augmentation to enhance implant predictability and esthetics: ‘The perio-esthetic approach’. J. Indian Soc. Periodontol. 2011, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Seibert, J.S. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part II. Prosthetic/periodontal interrelationships. Compend. Contin. Educ. Dent. 1983, 4, 549–562. [Google Scholar] [PubMed]
- Kan, J.Y.K.; Rungcharassaeng, K.; Umezu, K.; Kois, J.C. Dimensions of Peri-Implant Mucosa: An Evaluation of Maxillary Anterior Single Implants in Humans. J. Periodontol. 2003, 74, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Tolstunov, L. Horizontal Alveolar Ridge Augmentation in Implant Dentistry: A Surgical Manual; John Wiley & Sons: San Francisco, CA, USA, 2015; ISBN 9781119019886. [Google Scholar]
- Shalabi, M.M.; Wolke, J.G.C.; de Ruijter, A.J.E.; Jansen, J.A. A Mechanical Evaluation of Implants Placed With Different Surgical Techniques Into the Trabecular Bone of Goats. J. Oral Implantol. 2007, 33, 51–58. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Wolke, J.G.C.; Jansen, J.A. Influence of the surgical technique and surface roughness on the primary stability of an implant in artificial bone with a density equivalent to maxillary bone: A laboratory study. Clin. Oral Implant. Res. 2009, 20, 327–332. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Cho, S.C.; Wallace, S.S. The Effect of Inter-Implant Distance on the Height of Inter-Implant Bone Crest. J. Periodontol. 2000, 71, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Miloro, M.; Peterson, L.J. Peterson’s Principles of Oral and Maxillofacial Surgery; People’s Medical Pub. House: Shelton, CT, USA, 2012; ISBN 1607951118. [Google Scholar]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Chan, M.F.; Närhi, T.O.; de Baat, C.; Kalk, W. Treatment of the atrophic edentulous maxilla with implant-supported overdentures: A review of the literature. Int. J. Prosthodont. 1998, 11, 7–15. [Google Scholar]
- Abou Neel, E.A.; Young, A.M. Antibacterial adhesives for bone and tooth repair. In Joining and Assembly of Medical Materials and Devices; Elsevier: Philadelphia, PA, USA, 2013; pp. 491–513. [Google Scholar]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Tian, X.; Cui, G.; Zhao, Y.; Yang, Q.; Yu, S.; Xing, G.; Zhang, B. Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials 2009, 30, 6276–6285. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zara, J.N.; Siu, R.K.; Lee, M.; Aghaloo, T.; Zhang, X.; Wu, B.M.; Gertzman, A.A.; Ting, K.; Soo, C. Nell-1 Enhances Bone Regeneration in a Rat Critical-Sized Femoral Segmental Defect Model. Plast. Reconstr. Surg. 2011, 127, 580–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemcovsky, C.E.; Artzi, Z.; Moses, O. Rotated palatal flap in immediate implant procedures. Clin. Oral Implant. Res. 2000, 11, 83–90. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The role of growth factors in the repair of bone. J. Bone Jt. Surg. 2002, 84, 1032–1044. [Google Scholar] [CrossRef]
- Florjanski, W.; Orzeszek, S.; Olchowy, A.; Grychowska, N.; Wieckiewicz, W.; Malysa, A.; Smardz, J.; Wieckiewicz, M. Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration. Polymers 2019, 11, 782. [Google Scholar] [CrossRef] [Green Version]
- Neiva, W.V.R. Giannobile Barrier Membrane—An Overview|Science Direct Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/barrier-membrane (accessed on 9 February 2020).
- Alman, B.A.; de Bari, A.; Krajbich, J.I. Massive allografts in the treatment of osteosarcoma and Ewing sarcoma in children and adolescents. J. Bone Jt. Surg. 1995, 77, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, S.K.; Thiyam, R. Management of infection following reconstruction in bone tumors. J. Clin. Orthop. Trauma 2015, 6, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Chan, C.K.; Patil, N.; Goodman, S.B. Cell therapy for bone regeneration-Bench to bedside. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89B, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Nkenke, E.; Schultze-Mosgau, S.; Radespiel-Tröger, M.; Kloss, F.; Neukam, F.W. Morbidity of harvesting of chin grafts: A prospective study. Clin. Oral Implant. Res. 2001, 12, 495–502. [Google Scholar] [CrossRef]
- Misch, C.M.; Misch, C.E. The repair of localized severe ridge defects for implant placement using mandibular bone grafts. Implant Dent. 1995, 4, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.M.; Misch, C.E.; Resnik, R.R.; Ismail, Y.H. Reconstruction of maxillary alveolar defects with mandibular symphysis grafts for dental implants: A preliminary procedural report. Int. J. Oral Maxillofac. Implant. 1992, 7, 360–366. [Google Scholar]
- Clavero, J.; Lundgren, S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: Comparison of donor site morbidity and complications. Clin. Implant Dent. Relat. Res. 2003, 5, 154–160. [Google Scholar] [CrossRef]
- Blanchaert, R.H.; Harris, C.M. Microvascular Free Bone Flaps. Atlas Oral Maxillofac. Surg. Clin. 2005, 13, 151–171. [Google Scholar] [CrossRef]
- Torroni, A. Engineered Bone Grafts and Bone Flaps for Maxillofacial Defects: State of the Art. J. Oral Maxillofac. Surg. 2009, 67, 1121–1127. [Google Scholar] [CrossRef]
- Harii, K. Clinical application of free omental flap transfer. Clin. Plast. Surg. 1978, 5, 273–281. [Google Scholar]
- Schliephake, H. Revascularized tissue transfer for the repair of complex midfacial defects in oncologic patients. J. Oral Maxillofac. Surg. 2000, 58, 1212–1218. [Google Scholar] [CrossRef]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5, S125–S127. [Google Scholar] [CrossRef]
- Wei, P.-C.; Laurell, L.; Geivelis, M.; Lingen, M.W.; Maddalozzo, D. Acellular Dermal Matrix Allografts to Achieve Increased Attached Gingiva. Part 1. A Clinical Study. J. Periodontol. 2000, 71, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Wagshall, E.; Lewis, Z.; Babich, S.B.; Sinensky, M.C.; Hochberg, M. Acellular dermal matrix allograft in the treatment of mucogingival defects in children: Illustrative case report. J. Dent. Child. 2002, 69, 39–43. [Google Scholar]
- Kimelman Bleich, N.; Kallai, I.; Lieberman, J.R.; Schwarz, E.M.; Pelled, G.; Gazit, D. Gene therapy approaches to regenerating bone. Adv. Drug Deliv. Rev. 2012, 64, 1320–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G.; et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures. J. Bone Jt. Surg. 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Hernigou, P. Bone transplantation and tissue engineering. Part II: Bone graft and osteogenesis in the seventeenth, eighteenth and nineteenth centuries (Duhamel, Haller, Ollier and MacEwen). Int. Orthop. 2015, 39, 193–204. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury 2011, 42, S56–S63. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, A.S.; Mikos, A.G. Tissue Engineering Strategies for Bone Regeneration. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 94, pp. 1–22. [Google Scholar]
- Thorpe, A.A.; Creasey, S.; Sammon, C.; Le Maitre, C.L. Hydroxyapatite nanoparticle injectable hydrogel scaffold to support osteogenic differentiation of human mesenchymal stem cells. Eur. Cell. Mater. 2016, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
- McMahon, R.E.; Wang, L.; Skoracki, R.; Mathur, A.B. Development of nanomaterials for bone repair and regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O.C. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C. Nanomaterial-based bone regeneration. Nanoscale 2017, 9, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Huang, J.; Barber, Z.H.; Best, S.M.; Bonfield, W. Surface modification of magnetron-sputtered hydroxyapatite thin films via silicon substitution for orthopaedic and dental applications. Surf. Coat. Technol. 2011, 205, 3472–3477. [Google Scholar] [CrossRef]
- Notodihardjo, F.Z.; Kakudo, N.; Kushida, S.; Suzuki, K.; Kusumoto, K. Bone regeneration with BMP-2 and hydroxyapatite in critical-size calvarial defects in rats. J. Cranio-Maxillofac. Surg. 2012, 40, 287–291. [Google Scholar] [CrossRef]
- Ahmed, R.; Faisal, N.H.; Paradowska, A.M.; Fitzpatrick, M.E.; Khor, K.A. Neutron diffraction residual strain measurements in nanostructured hydroxyapatite coatings for orthopaedic implants. J. Mech. Behav. Biomed. Mater. 2011, 4, 2043–2054. [Google Scholar] [CrossRef]
- Habibah, T.U.; Salisbury, H.G. Hydroxyapatite Dental Material; StatPearls Publishing: Petersburg, FL, USA, 2020. [Google Scholar]
- Pan, S.; Yu, H.; Yang, X.; Yang, X.; Wang, Y.; Liu, Q.; Jin, L.; Yang, Y. Application of Nanomaterials in Stem Cell Regenerative Medicine of Orthopedic Surgery. J. Nanomater. 2017, 2017, 1985942. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef] [Green Version]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Al-Hazmi, F.; Alnowaiser, F.; Al-Ghamdi, A.A.; Al-Ghamdi, A.A.; Aly, M.M.; Al-Tuwirqi, R.M.; El-Tantawy, F. A new large—Scale synthesis of magnesium oxide nanowires: Structural and antibacterial properties. Superlattices Microstruct. 2012, 52, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauth, A.; Lane, J.; Watson, J.T.; Giannoudis, P. Bone Graft Substitution and Augmentation. J. Orthop. Trauma 2015, 29, S34–S38. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, Y.W.; Yang, K.H.; Kim, S.B.; Yoo, M.J.; Han, S.K.; Im, S.A.; Won, Y.D.; Sung, Y.B.; Jeon, T.S.; et al. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast(Ossron) injection to treat fractures. BMC Musculoskelet Disord. 2009, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leupold, J.A.; Barfield, W.R.; An, Y.H.; Hartsock, L.A. A comparison of ProOsteon, DBX, and collagraft in a rabbit model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 79B, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kukkar, N.; Sharif, K.; Main, B.J.; Albers, C.E., III. Bone graft substitutes for spine fusion: A brief review. World J. Orthop. 2015, 6, 449. [Google Scholar] [CrossRef]
- Van Vugt, T.A.G.; Geurts, J.; Arts, J.J. Clinical Application of Antimicrobial Bone Graft Substitute in Osteomyelitis Treatment: A Systematic Review of Different Bone Graft Substitutes Available in Clinical Treatment of Osteomyelitis. Biomed Res. Int. 2016, 2016, 6984656. [Google Scholar] [CrossRef] [Green Version]
- Basha, R.Y.; Sampath Kumar, T.S.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Dahabreh, Z.; Panteli, M.; Pountos, I.; Howard, M.; Campbell, P.; Giannoudis, P.V. Ability of bone graft substitutes to support the osteoprogenitor cells: An in-vitro study. World J. Stem Cells 2014, 6, 497. [Google Scholar] [CrossRef]
- Bojar, W.; Ciach, T.; Kucharska, M.; Maurin, J.; Gruber, B.; Krzysztoń-Russjan, J.; Bubko, I.; Anuszewska, E. Cytotoxicity Evaluation and Crystallochemical Analysis of a Novel and Commercially Available Bone Substitute Material. Adv. Clin. Exp. Med. 2015, 24, 511–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurencin, C.; Khan, Y.; El-Amin, S.F. Bone graft substitutes. Expert Rev. Med. Devices 2006, 3, 49–57. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattimani, V.; Lingamaneni, K.P.; Chakravarthi, P.S.; Kumar, T.S.S.; Siddharthan, A. Eggshell-Derived Hydroxyapatite. J. Craniofac. Surg. 2016, 27, 112–117. [Google Scholar] [CrossRef]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L.; Morsi, Y.; Zou, Q.; Wang, Y.; Gao, S.; Li, Y. Hydroxyapatite/polyamide66 porous scaffold with an ethylene vinyl acetate surface layer used for simultaneous substitute and repair of articular cartilage and underlying bone. Appl. Surf. Sci. 2011, 257, 9888–9894. [Google Scholar] [CrossRef]
- Tavakol, S.; Nikpour, M.R.; Amani, A.; Soltani, M.; Rabiee, S.M.; Rezayat, S.M.; Chen, P.; Jahanshahi, M. Bone regeneration based on nano-hydroxyapatite and hydroxyapatite/chitosan nanocomposites: An in vitro and in vivo comparative study. J. Nanoparticle Res. 2013, 15, 1373. [Google Scholar] [CrossRef]
- Fang, L.; Leng, Y.; Gao, P. Processing of hydroxyapatite reinforced ultrahigh molecular weight polyethylene for biomedical applications. Biomaterials 2005, 26, 3471–3478. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Feng, Q.-L.; Zhao, W.; Yu, B.; Tian, J.; Li, S.-J.; Lin, B.-M. In vivo bone regeneration with injectable chitosan/hydroxyapatite/collagen composites and mesenchymal stem cells. Front. Mater. Sci. 2011, 5, 301–310. [Google Scholar] [CrossRef]
- Wang, F.; Su, X.-X.; Guo, Y.-C.; Li, A.; Zhang, Y.-C.; Zhou, H.; Qiao, H.; Guan, L.-M.; Zou, M.; Si, X.-Q. Bone Regeneration by Nanohydroxyapatite/Chitosan/Poly(lactide-co-glycolide) Scaffolds Seeded with Human Umbilical Cord Mesenchymal Stem Cells in the Calvarial Defects of the Nude Mice. Biomed. Res. Int. 2015, 2015, 261938. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, D.B.; Middleton, J.C.; Tannenbaum, R.; Wick, T.M. Mechanical properties and osteogenic potential of hydroxyapatite-PLGA-collagen biomaterial for bone regeneration. J. Biomater. Sci. Polym. Ed. 2016, 27, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.E.; Miller, A.H.; Wang, J. Collagen Scaffolds in Bone Sialoprotein-Mediated Bone Regeneration. Sci. World J. 2013, 2013, 812718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, K.-S.; Kao, S.-Y.; Wang, C.-Y.; Wang, Y.-J.; Wang, J.-P.; Hung, S.-C. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J. Biomed. Mater. Res. Part A 2010, 94, 6732013682. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef] [Green Version]
- Kalita, S.J.; Bhatt, H.A. Nanocrystalline hydroxyapatite doped with magnesium and zinc: Synthesis and characterization. Mater. Sci. Eng. C 2007, 27, 837–848. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, M.; Li, Z.; Casciano, D.; Khodakovskaya, M.V.; Chen, T.; Karmakar, A.; Dervishi, E.; Xu, Y.; Mustafa, T.; Watanabe, F.; et al. Nanostructural materials increase mineralization in bone cells and affect gene expression through miRNA regulation. J. Cell. Mol. Med. 2011, 15, 2297–2306. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.; Yen, H.-J.; Tsai, C.-L. The Response of Articular Chondrocytes to Type II Collagen–Au Nanocomposites. Artif. Organs 2007, 31, 854–868. [Google Scholar] [CrossRef]
- Kumar, V.B.; Khajuria, D.K.; Karasik, D.; Gedanken, A. Silver and gold doped hydroxyapatite nanocomposites for enhanced bone regeneration. Biomed. Mater. 2019, 14, 055002. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef] [Green Version]
- Qing, T.; Mahmood, M.; Zheng, Y.; Biris, A.S.; Shi, L.; Casciano, D.A. A genomic characterization of the influence of silver nanoparticles on bone differentiation in MC3T3-E1 cells. J. Appl. Toxicol. 2018, 38, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sato, Y.; Haniu, H.; Nomura, H.; Kobayashi, S.; Takanashi, S.; Okamoto, M.; Takizawa, T.; Aoki, K.; Usui, Y.; et al. A three-dimensional block structure consisting exclusively of carbon nanotubes serving as bone regeneration scaffold and as bone defect filler. PLoS ONE 2017, 12, e0172601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, N.; Haniu, H.; Usui, Y.; Aoki, K.; Hara, K.; Takanashi, S.; Shimizu, M.; Narita, N.; Okamoto, M.; Kobayashi, S.; et al. Safe Clinical Use of Carbon Nanotubes as Innovative Biomaterials. Chem. Rev. 2014, 114, 6040–6079. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Y.; Lai, Y.; Yang, W.; Jiao, F.; Zhang, H.; Ye, S.; Zhang, Q. Incorporation of carboxylation multiwalled carbon nanotubes into biodegradable poly(lactic-co-glycolic acid) for bone tissue engineering. Colloids Surf. B Biointerfaces 2011, 83, 367–375. [Google Scholar] [CrossRef]
- Saito, N.; Usui, Y.; Aoki, K.; Narita, N.; Shimizu, M.; Ogiwara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; Taruta, S.; et al. Carbon Nanotubes for Biomaterials in Contact with Bone. Curr. Med. Chem. 2008, 15, 523–527. [Google Scholar] [CrossRef]
- Venkatesan, J.; Pallela, R.; Kim, S.-K. Applications of Carbon Nanomaterials in Bone Tissue Engineering. J. Biomed. Nanotechnol. 2014, 10, 3105–3123. [Google Scholar] [CrossRef]
- Shimizu, M.; Kobayashi, Y.; Mizoguchi, T.; Nakamura, H.; Kawahara, I.; Narita, N.; Usui, Y.; Aoki, K.; Hara, K.; Haniu, H.; et al. Carbon Nanotubes Induce Bone Calcification by Bidirectional Interaction with Osteoblasts. Adv. Mater. 2012, 24, 2176–2185. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Lewis, J.S. Dendritic cells in the host response to implanted materials. Semin. Immunol. 2017, 29, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Bottoni, C.R.; Smith, E.L.; Shaha, J.; Shaha, S.S.; Raybin, S.G.; Tokish, J.M.; Rowles, D.J. Autograft Versus Allograft Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2015, 43, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Sher, F.; Rößler, R.; Brouwer, N.; Balasubramaniyan, V.; Boddeke, E.; Copray, S. Differentiation of Neural Stem Cells into Oligodendrocytes: Involvement of the Polycomb Group Protein Ezh2. Stem Cells 2008, 26, 2875–2883. [Google Scholar] [CrossRef]
- Biris, A.R.; Mahmood, M.; Lazar, M.D.; Dervishi, E.; Watanabe, F.; Mustafa, T.; Baciut, G.; Baciut, M.; Bran, S.; Ali, S.; et al. Novel Multicomponent and Biocompatible Nanocomposite Materials Based on Few-Layer Graphenes Synthesized on a Gold/Hydroxyapatite Catalytic System with Applications in Bone Regeneration. J. Phys. Chem. C 2011, 115, 18967–18976. [Google Scholar] [CrossRef]
- Crisan, L.; Crisan, B.V.; Bran, S.; Onisor, F.; Armencea, G.; Vacaras, S.; Lucaciu, O.P.; Mitre, I.; Baciut, M.; Baciut, G.; et al. Carbon-based nanomaterials as scaffolds in bone regeneration. Part. Sci. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- Cheng, X.; Wan, Q.; Pei, X. Graphene Family Materials in Bone Tissue Regeneration: Perspectives and Challenges. Nanoscale Res. Lett. 2018, 13, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbandi, A.; Gottardo, E.; Huff, J.; Stroscio, M.; Shokuhfar, T. A Review of the Cell to Graphene-Based Nanomaterial Interface. JOM 2018, 70, 566–574. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Weir, M.D.; Simon, C.G. Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dent. Mater. 2008, 24, 1212–1222. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Leeuwenburgh, S.C.G.; Li, Y.; Jansen, J.A. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2012, 18, 24–39. [Google Scholar] [CrossRef]
- Carbone, E.J.; Jiang, T.; Nelson, C.; Henry, N.; Lo, K.W.-H. Small molecule delivery through nanofibrous scaffolds for musculoskeletal regenerative engineering. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1691–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, S.; Vial, S.; Reis, R.L.; Oliveira, J.M. Nanoparticles for bone tissue engineering. Biotechnol. Prog. 2017, 33, 590–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Mikos, A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 2007, 28, 3217–3227. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Feng, C.; Quan, J.; Wang, Z.; Wei, W.; Zang, S.; Kang, S.; Hui, G.; Chen, X.; Wang, Q. In situ controlled release of stromal cell-derived factor-1α and antimiR-138 for on-demand cranial bone regeneration. Carbohydr. Polym. 2018, 182, 215–224. [Google Scholar] [CrossRef]
- Silva, R.; Fabry, B.; Boccaccini, A.R. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials 2014, 35, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Imaizumi, H.; Kamakura, S.; Katagiri, T. Bone Regeneration by Synthetic Octacalcium Phosphate and its Role in Biological Mineralization. Curr. Med. Chem. 2008, 15, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Yang, D.-A.; Zhang, N.; Ji, C.-G.; Zhu, L.; Zhang, T. Poly(propylene fumarate)/(calcium sulphate/β-tricalcium phosphate) composites: Preparation, characterization and in vitro degradation. Acta Biomater. 2009, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, H.N.; Lim, K.-T.; Kim, Y.; Seonwoo, H.; Park, S.H.; Lim, H.J.; Kim, D.-H.; Suh, K.-Y.; Choung, P.-H.; et al. Designing nanotopographical density of extracellular matrix for controlled morphology and function of human mesenchymal stem cells. Sci. Rep. 2013, 3, 3552. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; van Blitterswijk, C.A.; Feng, Q.; Cui, F.; Watari, F. The effect of calcium phosphate microstructure on bone-related cells in vitro. Biomaterials 2008, 29, 3306–3316. [Google Scholar] [CrossRef]
- Yao, X.; Peng, R.; Ding, J. Effects of aspect ratios of stem cells on lineage commitments with and without induction media. Biomaterials 2013, 34, 930–939. [Google Scholar] [CrossRef]
- Janeczek, M.; Szymczyk, P.; Dobrzynski, M.; Parulska, O.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Zywicka, B.; Ziolkowski, G.; Marycz, K.; et al. Influence of surface modifications of a nanostructured implant on osseointegration capacity—Preliminary in vivo study. RSC Adv. 2018, 8, 15533–15546. [Google Scholar] [CrossRef] [Green Version]
- Paz, A.G.; Maghaireh, H.; Mangano, F.G. Stem Cells in Dentistry: Types of Intra- and Extraoral Tissue-Derived Stem Cells and Clinical Applications. Stem Cells Int. 2018, 2018, 4313610. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—Is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Felice, P.; Pistilli, R.; Lizio, G.; Pellegrino, G.; Nisii, A.; Marchetti, C. Inlay versus Onlay Iliac Bone Grafting in Atrophic Posterior Mandible: A Prospective Controlled Clinical Trial for the Comparison of Two Techniques. Clin. Implant Dent. Relat. Res. 2009, 11, e69–e82. [Google Scholar] [CrossRef]
- Khojasteh, A.; Behnia, H.; Dashti, S.G.; Stevens, M. Current Trends in Mesenchymal Stem Cell Application in Bone Augmentation: A Review of the Literature. J. Oral Maxillofac. Surg. 2012, 70, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Slater, B.J.; Kwan, M.D.; Gupta, D.M.; Panetta, N.J.; Longaker, M.T. Mesenchymal cells for skeletal tissue engineering. Expert Opin. Biol. Ther. 2008, 8, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Kubo, T.; Doi, K.; Hayashi, K.; Morita, K.; Matsuura, A.; Teixeira, E.R.; Akagawa, Y. Comparative evaluation of bone regeneration using spherical and irregularly shaped granules of interconnected porous hydroxylapatite. A beagle dog study. J. Prosthodont. Res. 2011, 55, 104–109. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Ahn, H.H.; Kim, K.S.; Lee, J.H.; Lee, J.Y.; Kim, B.S.; Lee, I.W.; Chun, H.J.; Kim, J.H.; Lee, H.B.; Kim, M.S. In Vivo Osteogenic Differentiation of Human Adipose-Derived Stem Cells in an Injectable In Situ –Forming Gel Scaffold. Tissue Eng. Part A 2009, 15, 1821–1832. [Google Scholar] [CrossRef]
- Meijer, G.J.; de Bruijn, J.D.; Koole, R.; van Blitterswijk, C.A. Cell based bone tissue engineering in jaw defects. Biomaterials 2008, 29, 3053–3061. [Google Scholar] [CrossRef]
- Gregory, C.A.; Prockop, D.J.; Spees, J.L. Non-hematopoietic bone marrow stem cells: Molecular control of expansion and differentiation. Exp. Cell Res. 2005, 306, 330–335. [Google Scholar] [CrossRef]
- Williams, A.R.; Hare, J.M. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011, 109, 923–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [Green Version]
- De Bari, C.; dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawadi, G.; Vayssière, B.; Dunn, F.; Baron, R.; Roman-Roman, S. BMP-2 Controls Alkaline Phosphatase Expression and Osteoblast Mineralization by a Wnt Autocrine Loop. J. Bone Miner. Res. 2003, 18, 1842–1853. [Google Scholar] [CrossRef]
- Bruder, S.P.; Jaiswal, N.; Haynesworth, S.E. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell. Biochem. 1997, 64, 278–294. [Google Scholar] [CrossRef]
- Cancedda, R.; Mastrogiacomo, M.; Bianchi, G.; Derubeis, A.; Muraglia, A.; Quarto, R. Bone marrow stromal cells and their use in regenerating bone. Novartis Found. Symp. 2003, 249, 133–143, discussion 143-7, 170–174, 239–41. [Google Scholar]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [Green Version]
- Ashton, B.A.; Allen, T.D.; Howlett, C.R.; Eaglesom, C.C.; Hattori, A.; Owen, M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin. Orthop. Relat. Res. 1980, 151, 294–307. [Google Scholar] [CrossRef]
- Pereira, R.F.; Halford, K.W.; O’Hara, M.D.; Leeper, D.B.; Sokolov, B.P.; Pollard, M.D.; Bagasra, O.; Prockop, D.J. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Natl. Acad. Sci. USA 1995, 92, 4857–4861. [Google Scholar] [CrossRef] [Green Version]
- Diefenderfer, D.L.; Osyczka, A.M.; Reilly, G.C.; Leboy, P.S. BMP responsiveness in human mesenchymal stem cells. Connect. Tissue Res. 2003, 44, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.M.; Glowacki, J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J. Cell. Biochem. 2001, 82, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Zins, J.E.; Whitaker, L.A. Membranous vs endochondral bone autografts: Implications for craniofacial reconstruction. Surg. Forum 1979, 30, 521–523. [Google Scholar]
- Koole, R.; Bosker, H.; van der Dussen, F.N. Late secondary autogenous bone grafting in cleft patients comparing mandibular (ectomesenchymal) and iliac crest (mesenchymal) grafts. J. Craniomaxillofac. Surg. 1989, 17, 28–30. [Google Scholar] [CrossRef]
- Mashimo, T.; Sato, Y.; Akita, D.; Toriumi, T.; Namaki, S.; Matsuzaki, Y.; Yonehara, Y.; Honda, M. Bone marrow-derived mesenchymal stem cells enhance bone marrow regeneration in dental extraction sockets. J. Oral Sci. 2019, 61, 284–293. [Google Scholar] [CrossRef] [Green Version]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human Mesenchymal Stem Cells Differentiate to a Cardiomyocyte Phenotype in the Adult Murine Heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef]







| Material | Application | Merit | Reference |
|---|---|---|---|
| Autograft | Spine fusion | Gold standard | [124,125] |
| Allograft | Craniofacial bone injury | Osteoinductive, osteoconductive | [126] |
| BMP | Open tibial fractures | Osteoinduction | [127] |
| Bioactive glass | Osteomyelitis | Anti-infective carrier | [128] |
| Composites | Femoral or cancellous bone defects | Biocompatible, tunable physiochemical properties | [129,130] |
| Synthetic polymers | Spine fusion, loading-bearing sites | Controlled degradation, mechanical strength | [131,132] |
| Natural polymers | Spinal fusion | Flexible, biocompatible, and biodegradable | [133] |
| Ceramic | Craniofacial bone defect | Biodegradable, osteointegrative, osteoconductive | [134] |
| Glass-ceramic | Femoral | Osteogenic | [135] |
| Resorbable Membranes | Non-Resorbable Membranes |
|---|---|
| Polylactic | Cellulose acetate filter |
| Polylactic/polyglycolic | PTFE |
| PL,PG and trimethylcarbonate | ePTFE |
| PG and TMC | Titanium mesh |
| Polyethylene glycol | Ethylene cellulose |
| Collagen | Rubber dam |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, W.; Dobrzynski, M.; Rybak, Z.; Szymonowicz, M.; Wiglusz, R.J. Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process. Nanomaterials 2020, 10, 1216. https://doi.org/10.3390/nano10061216
Zakrzewski W, Dobrzynski M, Rybak Z, Szymonowicz M, Wiglusz RJ. Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process. Nanomaterials. 2020; 10(6):1216. https://doi.org/10.3390/nano10061216
Chicago/Turabian StyleZakrzewski, Wojciech, Maciej Dobrzynski, Zbigniew Rybak, Maria Szymonowicz, and Rafal J. Wiglusz. 2020. "Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process" Nanomaterials 10, no. 6: 1216. https://doi.org/10.3390/nano10061216