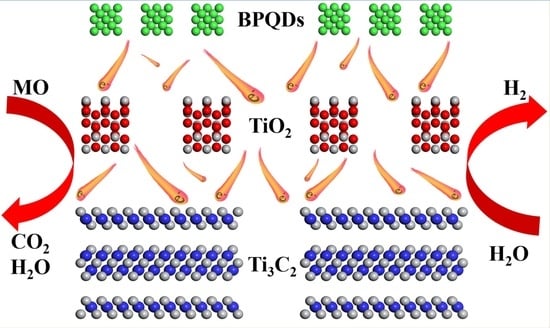
Construction of BPQDs/Ti3C2@TiO2 Composites with Favorable Charge Transfer Channels for Enhanced Photocatalytic Activity under Visible Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of BPQDs/Ti3C2@TiO2 Composites
2.3. Photocatalytic Degradation Reaction
2.4. Photocatalytic Hydrogen Evolution Reaction
2.5. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. New non-oxide photocatalysts designed for overall water splitting under visible Light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of eater at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Pan, J.; Lu, G.Q.M.; Cheng, H. Visible light responsive nitrogen doped anatase TiO2 sheets with dominant {001} facets derived from TiN. J. Am. Chem. Soc. 2009, 131, 12868–12869. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Wang, W.; Jaroniec, M. Nitrogen self-doped nanosized TiO2 sheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem. Commun. 2011, 47, 6906–6908. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Lu, N.; Hua, R.; Dong, B. Prolonging charge-separation states by doping lanthanide-ions into {001}/{101} facets-coexposed TiO2 nanosheets for enhancing photocatalytic H2 evolution. Chin. J. Catal. 2019, 40, 413–423. [Google Scholar] [CrossRef]
- Gahlot, S.; Jeanneau, E.; Dappozze, F.; Guillard, C.; Mishra, S. Precursor-mediated synthesis of Cu2−xSe nanoparticles and its composites with TiO2 for improved photocatalysis. Dalton Trans. 2018, 47, 8897–8905. [Google Scholar] [CrossRef]
- Humayun, M.; Zada, A.; Li, Z.; Xie, M.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L. Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl. Catal. B Environ. 2016, 180, 219–226. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Zheng, Y.; Chen, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 nanocrystalline heterostructure: A wide spectrum responsive photocatalyst towards the highly efficient decomposition of gaseous benzene. Appl. Catal. B Environ. 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Amanchi, S.R.; Kumar, K.V.A.; Lakshminarayana, B.; Satyanarayana, G.; Subrahmanyam, C. Photocatalytic hydrogenation of nitroarenes: Supporting effect of CoOx on TiO2 nanoparticles. New J. Chem. 2019, 43, 748–754. [Google Scholar] [CrossRef]
- Ismail, A.A.; Abdelfattah, I.; Helal, A.; Al-Sayari, S.A.; Robben, L.; Bahnemann, D.W. Ease synthesis of mesoporous WO3-TiO2 nanocomposites with enhanced photocatalytic performance for photodegradation of herbicide imazapyr under visible light and UV illumination. J. Hazard. Mater. 2016, 307, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, A.K.L.; Shamaila, S.; Tian, B.; Chen, F.; Zhang, J. One step activation of WOx/TiO2 nanocomposites with enhanced photocatalytic activity. Appl. Catal. B Environ. 2009, 91, 397–405. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Xie, H.; Guo, J.; Lyu, H.; Li, Y.; Sun, Z.; Wang, H.; Guo, Z. Electrospun titania nanofibers segregated by graphene oxide for improved visible light photocatalysis. Appl. Catal. B Environ. 2017, 201, 470–478. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.; Ma, T.; Du, A.; Qiao, S. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [Green Version]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Ng, V.M.H.; Huang, H.; Zhou, K.; Lee, P.S.; Que, W.; Xu, J.Z.; Kong, L.B. Recent progress in layered transition metal carbides and/or nitrides (MXenes) and their composites: Synthesis and applications. J. Mater. Chem. A 2017, 5, 3039–3068. [Google Scholar]
- Liu, N.; Lu, N.; Su, Y.; Wang, P.; Quan, X. Fabrication of g-C3N4/Ti3C2 composite and its visible-light photocatalytic capability for ciprofloxacin degradation. Sep. Purif. Technol. 2019, 211, 782–789. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, R.; Li, Y.; Qi, J.; Wang, C.; Li, J.; Sun, X.; Wang, L. Sandwich-like Co3O4/MXene composite with enhanced catalytic performance for bisphenol A degradation. Chem. Eng. J. 2018, 347, 731–740. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for photocatalytic degradation of antiepileptic drug carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Sun, Y.; Zhang, Y.; Zhou, Y. Controlling interfacial contact and exposed facets for enhancing photocatalysis via 2D-2D heterostructures. Chem. Commun. 2015, 51, 8249–8252. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, X.; Ong, W.; Zhao, X.; Li, N. Surface and heterointerface engineering of 2D MXenes and their nanocomposites: Insights into electro- and photocatalysis. Chem 2019, 5, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Ye, M.; Wang, X.; Liu, E.; Ye, J.; Wang, D. Boosting the photocatalytic activity of P25 for carbon dioxide reduction using a surface-alkalinized titanium carbide MXene as co-catalyst. ChemSusChem 2018, 10, 1606–1611. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, D.; Sun, Y.; Meng, X.; Gao, Y.; Agnese, Y.D.; Chen, G.; Wang, X. g-C3N4/Ti3C2Tx (MXenes) composite with oxidized surface groups for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 9124–9131. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Z.; Ji, G.; Liang, Z.; Xue, Y.; Guo, Y.; Tian, J.; Wang, X.; Cui, H. 2D/2D/2D heterojunction of Ti3C2 MXene/MoS2 nanosheets/TiO2 nanosheets with exposed (001) facets toward enhanced photocatalytic hydrogen production activity. Appl. Catal. B Environ. 2019, 246, 12–20. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Xiao, T.; Yuan, X.; Zeng, G.; Tu, W.; Wu, S.; Lee, H.Y.; Tan, Y.Z.; Chew, J.W. Formation of quasi-core-shell In2S3/anatase TiO2@metallic Ti3C2Tx hybrids with favorable charge transfer channels for excellent visible-light-photocatalytic performance. Appl. Catal. B Environ. 2018, 233, 213–225. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Li, X.; Liu, Y.; Cao, Y.; Wang, H.; Yu, H.; Peng, F.; Zhang, L.; Zhang, B.; et al. High efficiency photocatalytic hydrogen production over ternary Cu/TiO2@Ti3C2Tx enabled by low-work-function 2D titanium carbide. Nano Energy 2018, 53, 97–107. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Li, X.; Zhang, Q.; Chen, Z.; Li, H. Nitrogen-doped carbon quantum dots/BiOBr ultrathin nanosheets: In situ strong coupling and improved molecular oxygen activation ability under visible light irradiation. ACS Sustain. Chem. Eng. 2016, 4, 136–146. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Liu, Z.; Tan, C.; Luo, Z.; Li, H.; Lin, J.; Sun, L.; Chen, W.; Xu, Z.; et al. Black phosphorus quantum dots. Angewandte 2015, 54, 3633–3657. [Google Scholar]
- Pan, L.; Zhu, X.; Sun, K.; Liu, Y.; Xie, X.; Ye, X. Molecular level distribution of black phosphorus quantum dots on nitrogen-doped graphene nanosheets for superior lithium storage. Nano Energy 2016, 30, 347–354. [Google Scholar] [CrossRef]
- Fu, N.; Huang, C.; Lin, P.; Zhu, M.; Li, T.; Ye, M.; Lin, S.; Zhang, G.; Du, J.; Liu, C.; et al. Black phosphorus quantum dots as dual-functional electron-selective materials for efficient plastic perovskite solar cells. J. Mater. Chem. A 2018, 6, 8886–8894. [Google Scholar] [CrossRef]
- Gu, W.; Pei, X.; Cheng, Y.; Zhang, C.; Zhang, J.; Yan, Y.; Ding, C.; Xian, Y. Black phosphorus quantum dots as the ratiometric fluorescence probe for trace mercury ion detection based on inner filter effect. ACS Sens. 2017, 2, 576–582. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, S.; Wang, P.; Yang, Y.; Li, Z.; Chen, D.; Yu, Z.; Zou, Z. Bandgap-tunable black phosphorus quantum dots: Visible-light-active photocatalysts. Chem. Commun. 2018, 54, 960–963. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Z.; Han, J.; Jin, Z.; Han, Y.; Wang, F.; Niu, Y.; Wu, Y.; Xu, Y. High-performance electrocatalytic conversion of N2 to NH3 using oxygen-vacancy-rich TiO2 in situ grown on Ti3C2Tx MXene. Adv. Energy Mater. 2019, 9, 1803406. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Chen, Y.; Hou, B.; Zhang, N.; Chen, B.; Yang, N.; Chen, K.; Li, J.; An, L. Fabrication of layered Ti3C2 with an accordion-like structure as a potential cathode material for high performance lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 7870–7876. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Zhang, Y.; Jin, D.; Tao, X.; Zhang, L. Graphene coupled Ti3C2 MXenes-derived TiO2 mesostructure: Promising sodium-ion capacitor anode with fast ion storage and long-term cycling. J. Mater. Chem. A 2018, 6, 1017–1027. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Ti3C2 MXenes with modified surface for high-performance electromagnetic absorption and shielding in the X-band. ACS Appl. Mater. Interfaces 2016, 8, 21011–21019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Sun, N.; Anasori, B.; Zhu, Q.; Liu, H.; Gogotsi, Y.; Xu, B. Self-assembly of transition metal oxide nanostructures on MXene nanosheets for fast and stable lithium storage. Adv. Mater. 2018, 30, 1707334. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, L.; Tang, Y.; Luo, S.; Liu, Y.; Zhang, S.; Zeng, Y.; Xu, Y. Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2015, 164, 1–9. [Google Scholar] [CrossRef]
- Bai, S.; Liu, H.; Sun, J.; Tian, Y.; Chen, S.; Song, J.; Luo, R.; Li, D.; Chen, A.; Liu, C. Improvement of TiO2 photocatalytic properties under visible light by WO3/TiO2 and MoO3/TiO2 composites. Appl. Surf. Sci. 2015, 338, 61–68. [Google Scholar] [CrossRef]
- Xing, M.; Wu, Y.; Zhang, J.; Chen, F. Effect of synergy on the visible light activity of B, N and Fe co-doped TiO2 for the degradation of MO. Nanoscale 2010, 2, 1233–1239. [Google Scholar] [CrossRef]
- Meng, Z.; Zhu, L.; Choi, J.; Chen, M.; Oh, W. Effect of Pt treated fullerene/TiO2 on the photocatalytic degradation of MO under visible light. J. Mater. Chem. 2011, 21, 7596–7603. [Google Scholar] [CrossRef]
- Zang, Y.; Li, L.; Xu, Y.; Zuo, Y.; Li, G. Hybridization of brookite TiO2 with g-C3N4: A visible-light-driven photocatalyst for As3+ oxidation, MO degradation and water splitting for hydrogen evolution. J. Mater. Chem. A 2014, 2, 15774–15780. [Google Scholar] [CrossRef]
- Ilkhechi, N.N.; Akbarpour, M.R.; Yavari, R.; Azar, Z. Sn4+ and La3+ co doped TiO2 nanoparticles and their optical, photocatalytic and antibacterial properties under visible light. J. Mater. Sci. Mater. Electron. 2017, 28, 16658–16664. [Google Scholar] [CrossRef]
- Shi, H.; Yu, Y.; Zhang, Y.; Feng, X.; Zhao, X.; Tan, H.; Khan, S.U.; Li, Y.; Wang, E. Polyoxometalate/TiO2/Ag composite nanofibers with enhanced photocatalytic performance under visible light. Appl. Catal. B Environ. 2018, 221, 280–289. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Rafiee, E.; Bahnemann, D.W. A novel L-Histidine (C, N) codoped-TiO2-CdS nanocomposite for efficient visible photo-degradation of recalcitrant compounds from wastewater. J. Hazard. Mater. 2019, 369, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Kou, T.; Zhang, L.; Zhai, S.; Wang, W.; Wang, Y. Dealloying induced N-doping in spindle-like porous rutile TiO2 for enhanced visible light photocatalytic activity. Corros. Sci. 2018, 137, 204–211. [Google Scholar] [CrossRef]
- Zargari, S.; Rahimi, R.; Ghaffarinejad, A.; Morsali, A. Enhanced visible light photocurrent response and photodegradation efficiency over TiO2-graphene nanocomposite pillared with tin porphyrin. J. Colloid Interface Sci. 2016, 466, 310–321. [Google Scholar] [CrossRef] [PubMed]










| Composite | Visible Light Source | Catalyst Mass | Dye Concentration | Degradation Efficiency | References |
|---|---|---|---|---|---|
| (Fe, N, B)-TiO2 | 1000 W‡tungsten halogen lamp | 70 mg | MO, (20 mg/L), 50 mL | 300 min, 73% | [47] |
| Pt-fullerene/TiO2 | 8 W halogen lamp | 50 mg | MO, (3.3 mg/L), 50 mL | 120 min, 52% | [48] |
| br-TiO2/g-C3N4 | 300 W Xe lamp | 100 mg | MO, (10 mg/L), 100 mL | 180 min, 55% | [49] |
| TiO2-Sn-La | 150 W Xe lamp | 80 mg | MO, (5 mg/L), 50 mL | 120 min, 99% | [50] |
| PMo12/TiO2/Ag | 300 W Xe lamp | 20 mg | MO, (20 mg/L), 20 mL | 120 min, 99% | [51] |
| L-Histidine (C, N codoped)-TiO2-CdS | 50 W LED arrays | 300 mg | MO, (10 mg/L), 200 mL | 120 min, 95% | [52] |
| In2S3/anatase TiO2 @Ti3C2Tx | 300 W Xe lamp | 60 mg | MO, (20 mg/L), 100 mL | 60 min, 90% | [32] |
| TiO2-graphene | 450 W Xe lamp | 30 mg | MO, (10 mg/L), 50 mL | 180 min, 99% | [53] |
| N-doped rutile TiO2 | 300 W Xe lamp | 50 mg | MO, (10 mg/L), 25 mL | 120 min, 92% | [54] |
| BPQDs/Ti3C2@TiO2 | 400 W metal halide lamp | 50 mg | MO, (10 mg/L), 50 mL | 60 min, 93% | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.; Sun, H.; Sui, H.; Liu, X. Construction of BPQDs/Ti3C2@TiO2 Composites with Favorable Charge Transfer Channels for Enhanced Photocatalytic Activity under Visible Light Irradiation. Nanomaterials 2020, 10, 452. https://doi.org/10.3390/nano10030452
Yao Z, Sun H, Sui H, Liu X. Construction of BPQDs/Ti3C2@TiO2 Composites with Favorable Charge Transfer Channels for Enhanced Photocatalytic Activity under Visible Light Irradiation. Nanomaterials. 2020; 10(3):452. https://doi.org/10.3390/nano10030452
Chicago/Turabian StyleYao, Ziyu, Huajun Sun, Huiting Sui, and Xiaofang Liu. 2020. "Construction of BPQDs/Ti3C2@TiO2 Composites with Favorable Charge Transfer Channels for Enhanced Photocatalytic Activity under Visible Light Irradiation" Nanomaterials 10, no. 3: 452. https://doi.org/10.3390/nano10030452