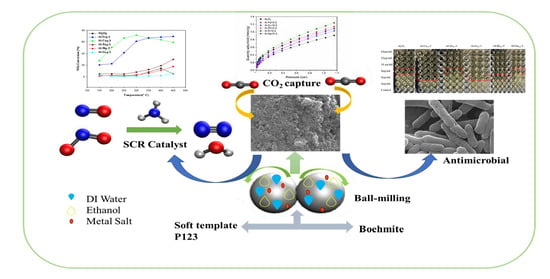
Exploring the Multifunctionality of Mechanochemically Synthesized γ-Alumina with Incorporated Selected Metal Oxide Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Information about the Materials Studied
2.2. Compositional Analysis of the Materials Studied
2.3. Low-Temperature N2 Sorption Analysis
2.4. Catalytic Tests
2.5. Carbon Dioxide Capture Study
2.6. Antimicrobial Activity against Pseudomonas aeruginosa
3. Experimental
3.1. Chemicals
3.2. Mechanochemical Synthesis of Metal Oxide-Incorporated γ-Al2O3
3.3. Measurements and Characterizations of γ-Alumina with Incorporated Metal Oxide Species
3.4. Calculations
3.5. Selective Catalytic Reduction of NO with Ammonia (NH3-SCR)
3.6. CO2 Capture Study
3.7. Determination of Minimum Inhibitory Concentrations
3.8. SEM Images of Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kresge, A.C.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, P.P.; Zhang, L.; Kinga, U.A.; Guo, Q.; Jiang, B.; Jaroniec, M. Development of nickel-incorporated MCM-41–carbon composites and their application in nitrophenol reduction. J. Mater. Chem. A Mater. 2019, 7, 9618–9628. [Google Scholar] [CrossRef]
- La-Salvia, N.; Lovón-Quintana, J.J.; Lovón, A.S.P.; Valença, G.P. Influence of aluminum addition in the framework of MCM-41 mesoporous molecular sieve synthesized by non-hydrothermal method in an alkali-free system. Mater. Res. 2017, 20, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Ajaikumar, S.; Pandurangan, A. Efficient synthesis of quinoxaline derivatives over ZrO2/MxOy (M = Al, Ga, In and La) mixed metal oxides supported on MCM-41 mesoporous molecular sieves. Appl. Catal. A Gen. 2009, 357, 184–192. [Google Scholar] [CrossRef]
- BoorboorAjdari, F.; Ostad, M.I.; Shahrak, M.N.; Ershadi, M.; Malek, S.S.; Ghasemi, F.; Ramakrishna, S. Investigating MCM-41/metal-organic framework nanocomposites as silicon-containing electrodes for supercapacitor. Surf. Interfaces 2022, 29, 101796–101807. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.X.; Luo, C.; Sun, L.D.; Zhang, Y.W.; Duan, W.T.; Liu, H.C.; Yan, C.H. Facile synthesis for ordered mesoporous γ-aluminas with high thermal stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Euro. J. Inorg. Chem. 2005, 7, 3393–3403. [Google Scholar] [CrossRef]
- Carstens, S.; Splith, C.; Enke, D. Sol-gel synthesis of α-Al2O3 with enhanced porosity via dicarboxylic acid templating. Sci. Rep. 2019, 9, 19982. [Google Scholar] [CrossRef] [Green Version]
- Lakade, S.H.; Harde, M.T.; Chattichalwadi, V.; Uttekar, P.S. Facile synthesis of mesoporous alumina using hexadecyltrimethylammonium bromide (HTAB) as template: Simplified sol-gel approach. IET Nanobiotechnol. 2019, 13, 834–841. [Google Scholar] [CrossRef]
- Benu, D.P.; Hardian, A.; Mukti, R.R.; Yuliarto, B.; Fukumitsu, N.; Ide, Y.; Yamauchi, Y.; Kaneti, Y.V.; Suendo, V. Reverse micelle-mediated synthesis of plate-assembled hierarchical three-dimensional flower-like gamma-alumina particles. Micropor. Mesopor. Mater. 2021, 321, 111055–111064. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Liu, C. Solvothermal synthesis of gamma aluminas and their structural evolution. Micropor. Mesopor. Mater. 2013, 167, 137–145. [Google Scholar] [CrossRef]
- Maziviero, F.V.; Medeiros, R.L.; Melo, D.M.; Macedo, H.P.; Oliveira, A.A.; Araujo, T.R. Synthesis of alumina by microwave-assisted combustion method using low fuel content and its use as catalytic support for dry reforming of methane. Mater. Chem. Phys. 2021, 264, 124408–124416. [Google Scholar] [CrossRef]
- Maruoka, H.; Kimura, T. An effective strategy to obtain highly porous alumina powders having robust and designable extra-large pores. Bull. Chem. Soc. Jpn. 2019, 92, 1859–1866. [Google Scholar] [CrossRef]
- Grant, S.M. Polymer Templating Synthesis, Adsorption and Structural Properties of Alumina-Based Ordered Mesoporous Materials. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2011. Available online: http://rave.ohiolink.edu/etdc/view?acc_num=kent1317593306 (accessed on 31 January 2023).
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. Cryst. Eng. Comm. 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Morris, S.M.; Fulvio, P.F.; Jaroniec, M. Ordered mesoporous alumina-supported metal oxides. J. Am. Chem. Soc. 2008, 130, 15210–15216. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, P.; Faure, B. Synthesis of highly porous alumina-based materials. Micropor. Mesopor. Mater. 2013, 181, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Daka, M.; Ferrara, M.; Bevilacqua, M.; Pengo, P.; Rajak, P.; Ciancio, R.; Montini, T.; Pasquato, L.; Fornasiero, P. Wet-Chemical Synthesis of Porous Multifaceted Platinum Nanoparticles for Oxygen Reduction and Methanol Oxidation Reactions. ACS Appl. Nano Mater. 2022, 5, 4710–4720. [Google Scholar] [CrossRef]
- Xu, Y.; Sprick, R.S.; Brownbill, N.J.; Blanc, F.; Li, Q.; Ward, J.W.; Ren, S.; Cooper, A.I. Bottom-up wet-chemical synthesis of a two-dimensional porous carbon material with high supercapacitance using a cascade coupling/cyclization route. J. Mater. Chem. A 2021, 9, 3303–3308. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical synthesis of catalytic materials. Chem.-A Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Paskevicius, M.; Webb, J.; Pitt, M.P.; Blach, T.P.; Hauback, B.C.; Gray, E.M.; Buckley, C.E. Mechanochemical synthesis of aluminium nanoparticles and their deuterium sorption properties to 2 kbar. J. Alloys Compd. 2009, 481, 595–599. [Google Scholar] [CrossRef]
- González Velázquez, V.J.; Vázquez, E.; Villajos, B.; Tolosana-Moranchel, Á.; Duran-Valle, C.; Faraldos, M.; Bahamonde, A. Eco-friendly mechanochemical synthesis of titania-graphene nanocomposites for pesticide photodegradation. Sep. Purif. Technol. 2022, 289, 1–32. Available online: http://hdl.handle.net/10578/29500 (accessed on 31 January 2023). [CrossRef]
- Basavalingiah, K.R.; Harishkumar, S.; Nagaraju, G.; Rangappa, D. Highly porous, honeycomb like Ag–ZnO nanomaterials for enhanced photocatalytic and photoluminescence studies: Green synthesis using Azadirachta indica gum. SN Appl. Sci. 2019, 1, 935. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Wu, Z.; Yip, A.C. Advances in the green synthesis of microporous and hierarchical zeolites: A short review. Catalysts 2019, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Szczęśniak, B.; Phuriragpitikhon, J. Choma, J.; Jaroniec, M. Mechanochemical synthesis of three-component graphene oxide/ordered mesoporous carbon/metal-organic framework composites. J. Colloid Interface Sci. 2020, 577, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Phuriragpitikhon, J.; Phinney, E.O.; Jaroniec, M. Potassium citrate-assisted eco-friendly synthesis of tannin-derived nitrogen-doped micro–mesoporous carbon microspheres. J. Mater. Sci. 2020, 55, 13716–13736. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327–2108357. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Tsuzuki, T.; McCormick, P.G.; Street, R. Ultrafine Cu particles prepared by mechanochemical process. J. Alloys Compd. 1996, 234, L1–L3. [Google Scholar] [CrossRef]
- Chalk, S.; McEwen, L. The IUPAC Gold Book. Chem. Int. 2017, 39, 25–30. [Google Scholar] [CrossRef]
- Takacs, L. Self-sustaining reactions induced by ball milling. Prog. Mater. Sci. 2002, 47, 355–414. [Google Scholar] [CrossRef]
- de Oliveira, P.F.; Torresi, R.M.; Emmerling, F.; Camargo, P.H. Challenges, and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Kersen, Ü. The gas-sensing potential of nanocrystalline SnO2 produced by a mechanochemical milling via centrifugal action. Appl. Phys. A Mater. Sci. Process 2002, 75, 559–563. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Recent advances in mechanochemical synthesis of mesoporous metal oxides. Mater. Adv. 2021, 2, 2510–2523. [Google Scholar] [CrossRef]
- Dubadi, R.; Huang, D.S.; Jaroniec, M. Mechanochemical synthesis of nanoparticles for potential antimicrobial properties. Materials 2023, 16, 1460. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Facile mechanochemical synthesis of highly mesoporous γ-Al2O3 using boehmite. Micropor. Mesopor. Mater. 2021, 312, 110792–110799. [Google Scholar] [CrossRef]
- Weidner, E.; Dubadi, D.; Samojeden, B.; Piasecki, A.; Jesionowski, T.; Jaroniec, M.; Ciesielczyk, F. Mechanochemical synthesis of alumina-based catalysts enriched with vanadia and lanthana for selective catalytic reduction of nitrogen oxides. Sci. Rep. 2022, 12, 21294. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.A.; Costa, M.J.; Zhang, L.; Ciesielczyk, F.; Jaroniec, M. One-pot synthesis of MeAl2O4 (Me = Ni, Co, or Cu) supported on γ-Al2O3 with ultralarge mesopores: Enhancing interfacial defects in γ-Al2O3 to facilitate the formation of spinel structures at lower temperatures. Chem. Mater. 2018, 30, 436–446. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ahn, W.S. CO2 capture using mesoporous alumina prepared by a sol–gel process. Chem. Eng. J. 2011, 166, 646–651. [Google Scholar] [CrossRef]
- Dassanayake, T.M.; Dassanayake, A.C.; Abeydeera, N.; Pant, B.D.; Jaroniec, M.; Kim, M.H.; Huang, S.D. An aluminum lining to the dark cloud of silver resistance: Harnessing the power of potent antimicrobial activity of γ-Alumina nanoparticles. Biomater. Sci. 2021, 9, 7996–8006. [Google Scholar] [CrossRef]
- Mutch, G.A.; Shulda, S.; McCue, A.J.; Menart, M.J.; Ciobanu, C.V.; Ngo, C.; Anderson, J.A.; Richards, R.M.; Vega-Maza, D. Carbon capture by metal oxides: Unleashing the potential of the (111) Facet. J. Am. Chem. Soc. 2018, 40, 4736–4742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, R.; Hoenes, K.; Meurle, T.; Hessling, M.; Spellerberg, B. The effects of violet and blue light irradiation on ESKAPE pathogens and human cells in presence of cell culture media. Sci. Rep. 2021, 11, 24473. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Lamouri, S.; Hamidouche, M.; Bouaouadja, N.; Belhouchet, H.; Garnier, V.; Fantozzi, G.; Trelkat, J.F. Control of the γ-alumina to α-alumina phase transformation for an optimized alumina densification. Bol. Soc. Esp. Ceram. Vidr. 2017, 56, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of Large Pore MCM-41 Molecular Sieves to Improve Pore Size Analysis Using Nitrogen Adsorption Measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Zhang, Y.; Shan, W.; Shi, X.; Yu, Y.; Zhang, R.; Meng, X.; Xiao, F.S.; He, H. Selective catalytic reduction of NOx with NH3: Opportunities and challenges of Cu-based small-pore zeolites. Natl. Sci. 2021, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]










| Sample | Notation * | Metal Salts Used in the Synthesis | Metal | Added Metal. (Wt.%) | Metal Oxide in the Sample (Wt.%) | XRF Data | |
|---|---|---|---|---|---|---|---|
| Al2O3 (Wt.%) | Metal Oxide (Wt.%) | ||||||
| Al2O3 | Al-3 | NA | NA | NA | NA | 100.00 | NA |
| Al2O3-Fe2O3 | Al-Fe5-3 | Fe(NO3)3·9H2O | Fe | 5 | 3.68 | 95.42 | 4.58 |
| Al2O3-Fe2O3 | Al-Fe10-3 | Fe(NO3)3·9H2O | Fe | 10 | 7.02 | 90.69 | 9.31 |
| Al2O3-CuO | Al-Cu5-3 | Cu(NO3)2·6H2O | Cu | 5 | 3.23 | 96.46 | 3.54 |
| Al2O3-CuO | Al-Cu10-3 | Cu(NO3)2·6H2O | Cu | 10 | 6.22 | 93.09 | 6.91 |
| Al2O3-ZnO | Al-Zn5-3 | Zn(NO3)2·6H2O | Zn | 5 | 3.19 | 96.73 | 3.27 |
| Al2O3-ZnO | Al-Zn10-3 | Zn(NO3)2·6H2O | Zn | 10 | 6.16 | 93.01 | 6.99 |
| Al2O3-Bi2O3 | Al-Bi5-3 | Bi(NO3)3·5H2O | Bi | 5 | 2.87 | 97.79 | 2.21 |
| Al2O3-Bi2O3 | Al-Bi10-3 | Bi(NO3)3·5H2O | Bi | 10 | 5.57 | 94.77 | 5.23 |
| Al2O3-Ga2O3 | Al-Ga5-3 | Ga(NO3)3 | Ga | 5 | 3.44 | 97.74 | 2.26 |
| Al2O3-Ga2O3 | Al-Ga10-3 | Ga(NO3)3 | Ga | 10 | 7.26 | 94.83 | 5.17 |
| Sample | SBET (m2·g−1) | Pore Diameter KJS (nm) | Single Point Pore Volume (cm3·g−1) | nCO2 (25 °C) (mmol·g−1) |
|---|---|---|---|---|
| Commercial γ-Al2O3 | 96 | 34.2 | 0.41 | - |
| Boehmite | 282 | 1.58 | 0.34 | - |
| Al-3 * | 266 | 17.7 | 0.86 | - |
| Al-3 | 320 | 15.2 | 0.96 | 0.85 |
| Al-Fe5-3 | 307 | 21.9 | 1.43 | 1.02 |
| Al-Fe10-3 | 246 | 23.9 | 1.20 | 1.07 |
| Al-Cu5-3 | 281 | 17.9 | 1.21 | 0.79 |
| Al-Cu10-3 | 277 | 29.2 | 1.27 | 0.96 |
| Al-Zn5-3 | 252 | 29.3 | 1.48 | 0.79 |
| Al-Zn10-3 | 255 | 31.1 | 1.32 | 1.01 |
| Al-Bi5-3 | 300 | 18.3 | 1.18 | 0.80 |
| Al-Bi10-3 | 320 | 17.5 | 1.08 | 1.16 |
| Al-Ga5-3 | 286 | 18.0 | 1.15 | 0.78 |
| Al-Ga10-3 | 280 | 18.2 | 1.15 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubadi, R.; Weidner, E.; Samojeden, B.; Jesionowski, T.; Ciesielczyk, F.; Huang, S.; Jaroniec, M. Exploring the Multifunctionality of Mechanochemically Synthesized γ-Alumina with Incorporated Selected Metal Oxide Species. Molecules 2023, 28, 2002. https://doi.org/10.3390/molecules28052002
Dubadi R, Weidner E, Samojeden B, Jesionowski T, Ciesielczyk F, Huang S, Jaroniec M. Exploring the Multifunctionality of Mechanochemically Synthesized γ-Alumina with Incorporated Selected Metal Oxide Species. Molecules. 2023; 28(5):2002. https://doi.org/10.3390/molecules28052002
Chicago/Turabian StyleDubadi, Rabindra, Ewelina Weidner, Bogdan Samojeden, Teofil Jesionowski, Filip Ciesielczyk, Songping Huang, and Mietek Jaroniec. 2023. "Exploring the Multifunctionality of Mechanochemically Synthesized γ-Alumina with Incorporated Selected Metal Oxide Species" Molecules 28, no. 5: 2002. https://doi.org/10.3390/molecules28052002