Dry Dosage Forms of Add-Value Bioactive Phenolic Compounds by Supercritical CO2-Assisted Spray-Drying
Abstract
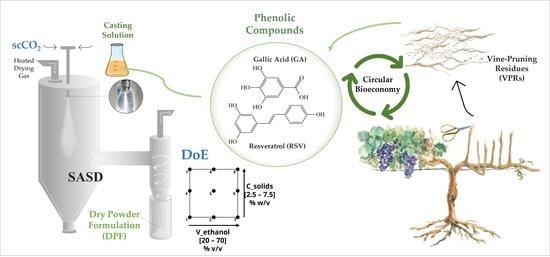
:1. Introduction
2. Results and Discussion
2.1. SASD Conditions
2.2. Co-Atomization with Bioactive Compounds
2.2.1. Statistical Analysis
2.2.2. Solid-State Characterization
2.2.3. Antioxidant Activity
2.2.4. In Vitro Aerodynamic Performance
2.2.5. In Vitro Phenolic Release
2.2.6. Quantification Tests
3. Materials and Methods
3.1. Material
3.2. Formulation and SASD Apparatus
3.3. Design of Experiments
3.4. Process Yield and Encapsulation Efficiency
3.5. Microparticle Characterization
3.5.1. Particle Size and Particle Size Distribution
3.5.2. Particle Morphology
3.5.3. X-ray Powder Diffraction (XRPD)
3.5.4. Specific Surface Area
3.5.5. Attenuated Total Reflection-Fourier Transform Infra-Red (ATR-FTIR)
3.5.6. Differential Scanning Calorimetry
3.5.7. Moisture Content
3.6. Antioxidant Activity
3.7. In Vitro Aerodynamic Performance
3.8. In Vitro Phenolic Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sánchez-Gómez, R.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. Reuse of Vine-Shoots Wastes for Agricultural Purposes. In Handbook of Grape Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–104. ISBN 978-0-12-809870-7. [Google Scholar]
- Maroun, R.G.; Rajha, H.N.; Vorobiev, E.; Louka, N. Emerging Technologies for the Recovery of Valuable Compounds From Grape Processing By-Products. In Handbook of Grape Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–181. ISBN 978-0-12-809870-7. [Google Scholar]
- International Organisation of Vine and Wine. Intergovernmental Organisation Statistical Report on World Vitiviniculture; OIV: Paris, France, 2019. [Google Scholar]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese Vine Shoot Wastes as Natural Resources of Bioactive Compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products; Academic Press: Cambridge, MA, USA, 2017; pp. 1–35. [Google Scholar]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral Valorization of Vine Pruning Residue by Sequential Autohydrolysis Stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, A.; Ysunza, F.; Beltrán-García, M.J.; Esqueda, M. Biodegradation of Viticulture Wastes by Pleurotus: A Source of Microbial and Human Food and Its Potential Use in Animal Feeding. J. Agric. Food Chem. 2002, 50, 2537–2542. [Google Scholar] [CrossRef]
- Dorosh, O.; Moreira, M.M.; Rodrigues, F.; Peixoto, A.F.; Freire, C.; Morais, S.; Delerue-Matos, C. Vine-Canes Valorisation: Ultrasound-Assisted Extraction from Lab to Pilot Scale. Molecules 2020, 25, 1739. [Google Scholar] [CrossRef] [PubMed]
- Lucia, G.; Daniel, H.; Jan, M.; Irena, K. Antioxidant Activity and Phenolic Content of Organic and Conventional Vine Cane Extracts. J. Food Sci. 2018, 36, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, Food and Pharma. Current Knowledge and Directions for Future Research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Tomiæ, J.; Stajiæ, Z.K.; Pesakoviæ, M. The Role of Berry Growing Technology on Bioactive Compound Improvement. In Phenolic Compounds: Structures, Uses and Health Benefits; Oliver, P., Villem, A., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2017. [Google Scholar]
- Dorosh, O.; Moreira, M.M.; Pinto, D.; F. Peixoto, A.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the Extraction Temperature Influence on Polyphenolic Profiles of Vine-Canes (Vitis Vinifera) Subcritical Water Extracts. Foods 2020, 9, 872. [Google Scholar] [CrossRef]
- Luque-Rodríguez, J.M.; Pérez-Juan, P.; Luque de Castro, M.D. Extraction of Polyphenols from Vine Shoots of Vitis Vinifera by Superheated Ethanol−Water Mixtures. J. Agric. Food Chem. 2006, 54, 8775–8781. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-Shoot Waste Aqueous Extracts for Re-Use in Agriculture Obtained by Different Extraction Techniques: Phenolic, Volatile, and Mineral Compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef]
- Jesus, M.S.; Genisheva, Z.; Romaní, A.; Pereira, R.N.; Teixeira, J.A.; Domingues, L. Bioactive Compounds Recovery Optimization from Vine Pruning Residues Using Conventional Heating and Microwave-Assisted Extraction Methods. Ind. Crops Prod. 2019, 132, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Alexandru, L.; Binello, A.; Mantegna, S.; Boffa, L.; Chemat, F.; Cravotto, G. Efficient Green Extraction of Polyphenols from Post-Harvested Agro-Industry Vegetal Sources in Piedmont. C. R. Chim. 2014, 17, 212–217. [Google Scholar] [CrossRef]
- Delgado-Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Pérez-Juan, P.M.; Luque de Castro, M.D. Comparison of Accelerated Methods for the Extraction of Phenolic Compounds from Different Vine-Shoot Cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar] [CrossRef]
- Max, B.; Salgado, J.M.; Cortés, S.; Domínguez, J.M. Extraction of Phenolic Acids by Alkaline Hydrolysis from the Solid Residue Obtained after Prehydrolysis of Trimming Vine Shoots. J. Agric. Food Chem. 2010, 58, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Wang, J. Ultrasound-Assisted Extraction of Five Isoflavones from Iris Tectorum Maxim. Sep. Purif. Technol. 2011, 78, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-Free Microwave Extraction of Essential Oil from Aromatic Herbs: Comparison with Conventional Hydro-Distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarian, B.; Paini, M.; Adami, R.; Perego, P.; Reverchon, E. Use of Supercritical Assisted Atomization to Produce Nanoparticles from Olive Pomace Extract. Innov. Food Sci. Emerg. Technol. 2017, 40, 2–9. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A Comparative Study of Physical Pretreatments for the Extraction of Polyphenols and Proteins from Vine Shoots. Int. Food Res. J. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds: A Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.K. Functional coatings and MicroEncapsulation: A General Perspective. In Functional Coatings by Polymer Microencapsulation; Ghosh, S.K., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Inactive Ingredient Database; Food and Drug Administration: Rockville, MD, USA, 2022.
- Penhasi, A. Microencapsulation of Probiotic Bacteria Using Thermo-Sensitive Sol-Gel Polymers for Powdered Infant Formula. J. Microencapsul. 2015, 32, 372–380. [Google Scholar] [CrossRef]
- Kabir, M.A.; Reo, J.P. Hydroxypropyl Cellulose. In Handbook of Pharmaceutical Excipients, 8th ed.; Sheskey, P.J., Cook, W.G., Cable, C.G., Eds.; Pharmaceutical Press: London, UK; The American Pharmacists Association: Washington, DC, USA, 2017. [Google Scholar]
- Tavares, M.; Cabral, R.P.; Costa, C.; Martins, P.; Fernandes, A.R.; Casimiro, T.; Aguiar-Ricardo, A. Development of PLGA Dry Powder Microparticles by Supercritical CO2-Assisted Spray-Drying for Potential Vaccine Delivery to the Lungs. J. Supercrit. Fluids 2017, 128, 235–243. [Google Scholar] [CrossRef]
- Temtem, M.; Silva, L.M.C.; Andrade, P.Z.; dos Santos, F.; da Silva, C.L.; Cabral, J.M.S.; Abecasis, M.M.; Aguiar-Ricardo, A. Supercritical CO2 Generating Chitosan Devices with Controlled Morphology. Potential Application for Drug Delivery and Mesenchymal Stem Cell Culture. J. Supercrit. Fluids 2009, 48, 269–277. [Google Scholar] [CrossRef]
- Silva, A.S.; Sousa, A.M.; Cabral, R.P.; Silva, M.C.; Costa, C.; Miguel, S.P.; Bonifácio, V.D.B.; Casimiro, T.; Correia, I.J.; Aguiar-Ricardo, A. Aerosolizable Gold Nano-in-Micro Dry Powder Formulations for Theragnosis and Lung Delivery. Int. J. Pharm. 2017, 519, 240–249. [Google Scholar] [CrossRef]
- Di Capua, A.; Bejarano, A.; Adami, R.; Reverchon, E. Preparation and Characterization of Chilean Propolis Coprecipitates Using Supercritical Assisted Atomization. Chem. Eng. Res. Des. 2018, 136, 776–785. [Google Scholar] [CrossRef]
- Altekar, M.; Homon, C.A.; Kashem, M.A.; Mason, S.W.; Nelson, R.M.; Patnaude, L.A.; Yingling, J.; Taylor, P.B. Assay Optimization: A Statistical Design of Experiments Approach. J. Assoc. Lab. Autom. 2006, 11, 33–41. [Google Scholar] [CrossRef]
- Cabral, R.P.; Sousa, A.M.L.; Silva, A.S.; Paninho, A.I.; Temtem, M.; Costa, E.; Casimiro, T.; Aguiar-Ricardo, A. Design of Experiments Approach on the Preparation of Dry Inhaler Chitosan Composite Formulations by Supercritical CO2-Assisted Spray-Drying. J. Supercrit. Fluids 2016, 116, 26–35. [Google Scholar] [CrossRef]
- Moura, C.; Casimiro, T.; Costa, E.; Aguiar-Ricardo, A. Optimization of Supercritical CO2-Assisted Spray Drying Technology for the Production of Inhalable Composite Particles Using Quality-by-Design Principles. Powder Technol. 2019, 357, 387–397. [Google Scholar] [CrossRef]
- Costa, C.; Nobre, B.; Matos, A.S.; Silva, A.S.; Casimiro, T.; Corvo, M.L.; Aguiar-Ricardo, A. Inhalable Hydrophilic Molecule-Loaded Liposomal Dry Powder Formulations Using Supercritical CO2—Assisted Spray-Drying. J. CO2 Util. 2021, 53, 101709. [Google Scholar] [CrossRef]
- Reverchon, E.; Porta, G.D.; Spada, A. Ampicillin Micronization by Supercritical Assisted Atomization. J. Pharm. Pharmacol. 2003, 55, 1465–1471. [Google Scholar] [CrossRef]
- Rahman, M.; Ahmad, S.; Tarabokija, J.; Parker, N.; Bilgili, E. Spray-Dried Amorphous Solid Dispersions of Griseofulvin in HPC/Soluplus/SDS: Elucidating the Multifaceted Impact of SDS as a Minor Component. Pharmaceutics 2020, 12, 197. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, N.D.; Guirguis, O.W. Macrostructure and Optical Studies of Hydroxypropyl Cellulose in Pure and Nano-Composites Forms. Results Phys. 2019, 15, 102637. [Google Scholar] [CrossRef]
- Sarode, A.; Wang, P.; Cote, C.; Worthen, D.R. Low-Viscosity Hydroxypropylcellulose (HPC) Grades SL and SSL: Versatile Pharmaceutical Polymers for Dissolution Enhancement, Controlled Release, and Pharmaceutical Processing. AAPS PharmSciTech 2013, 14, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-M.; Chang, Y.-C.; Cheng, L.-C.; Liu, C.-H.; Chang, S.C.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Preparation of Graphene-Embedded Hydroxypropyl Cellulose/Chitosan/Polyethylene Oxide Nanofiber Membranes as Wound Dressings with Enhanced Antibacterial Properties. Cellulose 2020, 27, 2651–2667. [Google Scholar] [CrossRef]
- Gómez-Carracedo, A.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; Concheiro, A. Chemical Structure and Glass Transition Temperature of Non-Ionic Cellulose Ethers. J. Therm. Anal. Calorim. 2003, 73, 587–593. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ghassami, E. Enhancement of Dissolution Rate of Fenofibrate by Spray Drying Technique: Comparison of Eudragit E-100, Solutol® HS15 and Hydroxypropyl Cellulose as Carriers. Farmacia 2015, 63, 433–445. [Google Scholar]
- Particle Analytical BET (Brunauer, Emmett and Teller). Available online: https://particle.dk/methods-analytical-laboratory/surface-area-bet-2/ (accessed on 5 November 2020).
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Dal Magro, C.; Aguiar, G.P.S.; Veneral, J.G.; dos Santos, A.E.; de Chaves, L.M.P.C.; Oliveira, J.V.; Lanza, M. Co-Precipitation of Trans-Resveratrol in PHBV Using Solution Enhanced Dispersion by Supercritical Fluids Technique. J. Supercrit. Fluids. 2017, 127, 182–190. [Google Scholar] [CrossRef]
- Alves, A. de C.S.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of Gallic Acid and Evaluation of Its Cytotoxicity and Antioxidant Activity. Mater. Sci. Eng. C. 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Ha, E.-S.; Sim, W.-Y.; Lee, S.-K.; Jeong, J.-S.; Kim, J.-S.; Baek, I.; Choi, D.H.; Park, H.; Hwang, S.-J.; Kim, M.-S. Preparation and Evaluation of Resveratrol-Loaded Composite Nanoparticles Using a Supercritical Fluid Technology for Enhanced Oral and Skin Delivery. Antioxidants 2019, 8, 554. [Google Scholar] [CrossRef] [Green Version]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of Food Grade Antioxidant in Natural Biopolymer by Electrospinning Technique: A Physicochemical Study Based on Zein-Gallic Acid System. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; González-Laredo, R.F.; Minjares-Fuentes, R.; Valadez-García, M.A.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation of Gallic Acid by Spray Drying with Aloe Vera Mucilage (Aloe Barbadensis Miller) as Wall Material. Ind. Crops Prod. 2019, 138, 111461. [Google Scholar] [CrossRef]
- Porto, I.C.C.M.; Nascimento, T.G.; Oliveira, J.M.S.; Freitas, P.H.; Haimeur, A.; França, R. Use of Polyphenols as a Strategy to Prevent Bond Degradation in the Dentin–Resin Interface. Eur. J. Oral Sci. 2018, 126, 146–158. [Google Scholar] [CrossRef]
- Pessoa, A.S.; Aguiar, G.P.S.; Vladimir Oliveira, J.; Bortoluzzi, A.J.; Paulino, A.; Lanza, M. Precipitation of Resveratrol-Isoniazid and Resveratrol-Nicotinamide Cocrystals by Gas Antisolvent. J. Supercrit. Fluids 2019, 145, 93–102. [Google Scholar] [CrossRef]
- Alexander, B.; Browse, D.J.; Reading, S.J.; Benjamin, I.S. A Simple and Accurate Mathematical Method for Calculation of the EC50. J. Pharmacol. Toxicol. Methods 1999, 41, 55–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Zhu, Y.; Xu, Z. Assessment of the Correlations between Reducing Power, Scavenging DPPH Activity and Anti-Lipid-Oxidation Capability of Phenolic Antioxidants. LWT-Food Sci Technol. 2015, 63, 569–574. [Google Scholar] [CrossRef]
- Aguiar, G.P.S.; Arcari, B.D.; Chaves, L.M.P.C.; Magro, C.D.; Boschetto, D.L.; Piato, A.L.; Lanza, M.; Oliveira, J.V. Micronization of Trans-Resveratrol by Supercritical Fluid: Dissolution, Solubility and in Vitro Antioxidant Activity. Ind. Crops Prod. 2018, 112, 1–5. [Google Scholar] [CrossRef]
- Silva, M.C.; Silva, A.S.; Fernandez-Lodeiro, J.; Casimiro, T.; Lodeiro, C.; Aguiar-Ricardo, A. Supercritical CO2-Assisted Spray Drying of Strawberry-like Gold-Coated Magnetite Nanocomposites in Chitosan Powders for Inhalation. Materials 2017, 10, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization Environmental Health Criteria 235; Dermal Absorption; WHO: Geneva, Switzerland, 2006.
- Zhang, X.-P.; Le, Y.; Wang, J.-X.; Zhao, H.; Chen, J.-F. Resveratrol Nanodispersion with High Stability and Dissolution Rate. LWT-Food Sci Technol. 2013, 50, 622–628. [Google Scholar] [CrossRef]
- Rahman, M.; Ahmad, S.; Tarabokija, J.; Bilgili, E. Roles of Surfactant and Polymer in Drug Release from Spray-Dried Hybrid Nanocrystal-Amorphous Solid Dispersions (HyNASDs). Powder Technol. 2020, 361, 663–678. [Google Scholar] [CrossRef]
- Robert, P.; García, P.; Reyes, N.; Chávez, J.; Santos, J. Acetylated Starch and Inulin as Encapsulating Agents of Gallic Acid and Their Release Behaviour in a Hydrophilic System. Food Chem. 2012, 134, 1–8. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and Solubility of Trans-Resveratrol Are Strongly Influenced by PH and Temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Narayan, P.; Porter III, W.W.; Brackhagen, M.; Tucker, C. Polymers and Surfactants. In Pharmaceutical Amorphous Solid Dispersions; Newman, A., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 42–84. ISBN 9781118455203. [Google Scholar]
- Dimer, F.A.; Ortiz, M.; Pohlmann, A.R.; Guterres, S.S. Inhalable Resveratrol Microparticles Produced by Vibrational Atomization Spray Drying for Treating Pulmonary Arterial Hypertension. J. Drug Deliv. Sci. Technol. 2015, 29, 152–158. [Google Scholar] [CrossRef]
- Cardoso, T.; Gonçalves, A.; Estevinho, B.N.; Rocha, F. Potential Food Application of Resveratrol Microparticles: Characterization and Controlled Release Studies. Powder Technol. 2019, 355, 593–601. [Google Scholar] [CrossRef]
- García, P.; Vergara, C.; Robert, P. Release Kinetic in Yogurt from Gallic Acid Microparticles with Chemically Modified Inulin. J. Food Sci. 2015, 80, C2147–C2152. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 19, 139–157. [Google Scholar] [CrossRef]
- European Pharmacopeia 7.0, Buffer Solutions; Council of Europe: Strasbourg, France, 2011; pp. 489–494.
- Ahmad, I.; Anwar, M.; Akhter, S.; Thakur, P.; Chawla, R.; Sharma, R.K.; Ali, A.; Ahmad, F.J. Supercritical Fluid Technology-Based Trans-Resveratrol SLN for Long Circulation and Improved Radioprotection. J. Pharm. Innov. 2016, 11, 308–322. [Google Scholar] [CrossRef]
- Badawy, S.I.F.; Gray, D.B.; Hussain, M.A. A Study on the Effect of Wet Granulation on Microcrystalline Cellulose Particle Structure and Performance. Pharm. Res. 2006, 23, 634–640. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopeia 7.0, Preparations for Inhalation: Aerodynamic Assessment of Fine Particles; Council of Europe: Strasbourg, France, 2010.
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]













| Exp. No. | C_Solids (%w/v) | Ethanol (%v/v) | Yield (%) | EE (%) | Dv,50 (µm) | Span |
|---|---|---|---|---|---|---|
| 1 | 2.5 | 20 | 49 ± 22 | 76 ± 1 | 24 ± 4 | 1.39 ± 0.06 |
| 2 | 2.5 | 45 | 44 ± 6 | 82 ± 12 | 22 ± 4 | 2.2 ± 0.3 |
| 3 | 2.5 | 70 | 53 ± 9 | 91 ± 2 | 28 ± 4 | 1.59 ± 0.05 |
| 4 | 5.0 | 20 | 52.5 ± 10.5 | 74 ± 8 | 19.64 ± 0.04 | 1.34 ± 0.03 |
| 5 | 5.0 | 45 | 55 ± 12 | 100 ± 2 | 18 ± 3 | 2.2 ± 0.4 |
| 6 | 5.0 | 70 | 36 ± 21 | 100 1 | 19.6 ± 0.6 | 1.8 ± 0.4 |
| 7 | 7.5 | 20 | 56 ± 2 | 88 ± 6 | 20 ± 1 | 1.33 ± 0.04 |
| 8 | 7.5 | 45 | 51.5 ± 0.5 | 100 1 | 15.6 ± 0.3 | 1.99 ± 0.02 |
| 9 | 7.5 | 70 | 72 ± 2 | 100 1 | 15 ± 1 | 2.8 ± 0.2 |
| Effect | p-Value | |
|---|---|---|
| Yield (%) | C_solid (%w/v) | n.s. 1 |
| Ethanol (%v/v) | n.s. 1 | |
| EE (%) | C_solid (%w/v) (L) | 0.0209 |
| Ethanol (%v/v) (L) | 0.0042 | |
| Dv,50 | C_solid (%w/v) (L) | 0.0079 |
| Ethanol (%v/v) | n.s. 1 | |
| span | C_solid (%w/v) | n.s. 1 |
| Ethanol (%v/v) (L) | 0.0069 | |
| Ethanol (%v/v) (Q) | 0.0347 | |
| C_solid (L) Ethanol (L) | 0.0284 |
| Exp. No. | C_Solids (%w/v) | Ethanol (%v/v) | Yield (%) | EE (%) | Dv,50 (µm) | Span |
|---|---|---|---|---|---|---|
| 1 | 2.5 | 20 | 42.5 ± 0.5 | 48 ± 4 | 22 ± 2 | 1.37 ± 0.08 |
| 2 | 2.5 | 45 | 21 ± 5 | 100 1 | 26 ± 8 | 2.0 ± 0.8 |
| 3 | 2.5 | 70 | 58.5 ± 3.5 | 88 ± 11 | 30 ± 1 | 1.8 ± 0.1 |
| 4 | 5.0 | 20 | 55 ± 3 | 65 ± 7 | 22 ± 4 | 1.5 ± 0.2 |
| 5 | 5.0 | 45 | 41 ± 15 | 94 ± 6 | 18 ± 4 | 2.2 ± 0.8 |
| 6 | 5.0 | 70 | 58 ± 2 | 100 1 | 23 ± 2 | 2.0 ± 0.2 |
| 7 | 7.5 | 20 | 50.5 ± 2.5 | 78 ± 7 | 21 ± 1 | 1.34 ± 0.02 |
| 8 | 7.5 | 45 | 58 ± 5 | 100 1 | 14 ± 2 | 2.3 ± 0.2 |
| 9 | 7.5 | 70 | 61 ± 2 | 96 ± 3 | 16 ± 2 | 1.9 ± 0.2 |
| Effect | p-Value | |
|---|---|---|
| Yield (%) | C_solid (%w/v) (L) | 0.0092 |
| Ethanol (%v/v) (Q) | 0.0075 | |
| C_solid (L) Ethanol (Q) | 0.0123 | |
| EE (%) | C_solid (%w/v) (L) | 0.0178 |
| Ethanol (%v/v) (L) | 0.0002 | |
| Ethanol (%v/v) (Q) | 0.0070 | |
| Dv,50 | C_solid (%w/v) (L) | 0.0162 |
| Ethanol (%v/v) | n.s. 1 | |
| span | C_solid (%w/v) | n.s. 1 |
| Ethanol (%v/v) | n.s. 1 |
| Sample | BET Specific Surface Area (m2/g) |
|---|---|
| HPC 2.5 %w/v | 1.95 |
| HPC 5.0 %w/v | 2.62 |
| HPC 7.5 %w/v | 2.08 |
| raw RSV | 2.19 |
| raw GA | 1.25 |
| RSV–DPF | 4.20 |
| GA–DPF | 2.04 |
| EC50 | |||||
|---|---|---|---|---|---|
| Sample | (µM) | (µg/mL) | Sample | (µM) | (µg/mL) |
| raw RSV | 528.99 | 120.74 | raw GA | 125.55 | 21.36 |
| DPF RSV/5.0/45 | 807.96 | 184.41 | DPF GA/5.0/45 | 163.40 | 37.29 |
| DPF RSV/7.5/20 | 672.43 | 153.47 | DPF GA/7.5/20 | 122.16 | 27.88 |
| DPF RSV/7.5/70 | 483.51 | 110.36 | DPF GA/7.5/70 | 117.28 | 26.77 |
| Higuchi | Korsmeyer–Peppas | ||||
|---|---|---|---|---|---|
| k (min−0.5) | R2 | k (min−n) | n | R2 | |
| RSV–DPF | 0.0055 † | 0.9975 † | 0.0001 ‡ | 1.1518 ‡ | 0.9932 ‡ |
| GA–DPF | 0.0507 | 0.9950 | 0.0051 | 0.9870 | 0.9948 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.; Anselmo, H.; Ferro, R.; Matos, A.S.; Casimiro, T.; Aguiar-Ricardo, A. Dry Dosage Forms of Add-Value Bioactive Phenolic Compounds by Supercritical CO2-Assisted Spray-Drying. Molecules 2022, 27, 2001. https://doi.org/10.3390/molecules27062001
Costa C, Anselmo H, Ferro R, Matos AS, Casimiro T, Aguiar-Ricardo A. Dry Dosage Forms of Add-Value Bioactive Phenolic Compounds by Supercritical CO2-Assisted Spray-Drying. Molecules. 2022; 27(6):2001. https://doi.org/10.3390/molecules27062001
Chicago/Turabian StyleCosta, Clarinda, Hugo Anselmo, Rita Ferro, Ana Sofia Matos, Teresa Casimiro, and Ana Aguiar-Ricardo. 2022. "Dry Dosage Forms of Add-Value Bioactive Phenolic Compounds by Supercritical CO2-Assisted Spray-Drying" Molecules 27, no. 6: 2001. https://doi.org/10.3390/molecules27062001