Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects
Abstract
:1. Introduction
2. Methodology
3. Cocoa Transformation
3.1. Cocoa Pre-Processing
3.2. Cocoa Processing
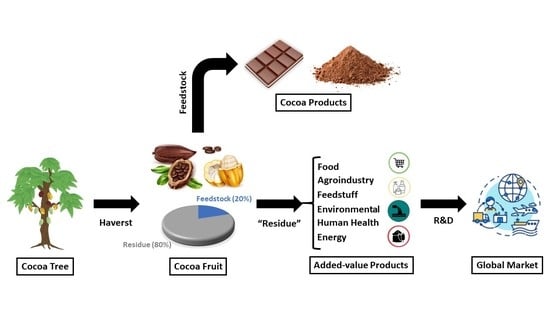
4. Cocoa By-Products
5. Nutritional Properties of Cocoa By-Products
5.1. Proteins and Amino Acids
5.2. Lipids
5.3. Dietary Fibres
5.4. Minerals and Vitamins
5.5. Phenolic and Antioxidant Compounds
6. Applications
6.1. Food
6.2. Agroindustry and Feedstuff
6.3. Environmental
6.4. Human Health
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afoakwa, E.O. Chocolate Science and Technology, 2nd ed.; Wiley-Blackwell Publishers: Chichester, UK, 2016; ISBN 978-1-118-91378-9. [Google Scholar]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomahb, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Queiroga, V.P.; Gomes, J.P.; Melo, B.A.; Alburquerque, E.M.B. Cacau (Theobroma cacao L.) Orgânico Sombreado Tecnologias de Plantio e Produção da Amêndoa Fina, 1st ed.; AREPB: Campina Grande, Brazil, 2021; ISBN 978-65-87070-08-7. [Google Scholar]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of Aromatic Compounds Precursors during Fermentation of Criollo and Forastero Cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 166, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, M.; Conti, A. Theobroma cacao L., the food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- ICCO—International Cocoa Organization Annual Report 2018/2019. Available online: https://www.icco.org/statistics/#production (accessed on 28 July 2021).
- Cruz, C.L.C.V. Melhoramento do Sabor da Amêndoa de Cacau Através de Tratamento Térmico em Forno Convencional e Microondas. Ph.D. Thesis, Universidade Federal de Campinas, Campinhas, Brazil, 2002. [Google Scholar]
- Beckett, S.T. The Science of Chocolate, 2nd ed.; RSC Publishing: Cambridge, UK, 2008; ISBN 978-0-85404-970-7. [Google Scholar]
- Efraim, P.; Alves, A.B.; Jardim, D.C.P. Polifenóis em cacau e derivados: Teores, fatores de variação e efeitos na saúde. Braz. J. Food Technol. 2011, 14, 181–201. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Relationship of sensory and instrumental aroma measurements of dark chocolate as influenced by fermentation method, roasting and conching conditions. J. Food Sci. Technol. 2013, 50, 909–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckett, S.T.; Fowler, M.S.; Ziegler, G.R. Industrial Chocolate Manufacture and Use, 4th ed.; John Willey & Sons: Chichester, UK, 2009; ISBN 978-1405139496. [Google Scholar]
- Lopes, A.S. Estudo Químico e Nutricional de Amêndoa de Cacau (Theobroma cacao L.) e Cupuaçu (Theobroma grandiflorum Schum) em Função do Processamento. Ph.D. Thesis, Universidade Federal de Campinas, Campinhas, Brazil, 2000. [Google Scholar]
- Lopes, A.S.; Garcia, N.H.P.; Vasconcelos, M.A.M. Avaliação das condições de torração após a fermentação da amêndoas de cupuaçu (Theobroma grandiflorum Schum) e cacau (Theobroma cacao L.). Braz. J. Food Technol. 2003, 6, 309–316. [Google Scholar]
- Silva, R.O. Utilização dos Resíduos Sólidos da Indústria Cacaueira para a Produção de Etanol. Ph.D. Thesis, Universidade Federal do Espírito Santo, Vitória, Brazil, 2018. [Google Scholar]
- Bonvehí, J.S.; Coll, F.V. Protein quality assessment in cocoa husk. Food Res. Int. 1999, 32, 201–208. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; de Mello Castanho Amboni, R.D.; de Oliveira Petkowicz, C.L. Cacao pod husks (Theobroma cacao L.): Composition and hot-water-soluble pectins. Ind. Crops Prod. 2011, 34, 1173–1181. [Google Scholar] [CrossRef]
- Anvoh, K.Y.B.; Bi, A.Z.; Gnakri, D. Production and characterization of juice from mucilage of cocoa beans and its transformation into marmalade. Pak. J. Nutr. 2009, 8, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Analytical dataset of Ecuadorian cocoa shells and beans. Data Br. 2019, 22, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Boateng, C.; Lee, K.T. The potential of using cocoa pod husks as green solid base catalysts for the transesterification of soybean oil into biodiesel: Effects of biodiesel on engine performance. Chem. Eng. J. 2013, 220, 395–401. [Google Scholar] [CrossRef]
- Mansur, D.; Tago, T.; Masuda, T.; Abimanyu, H. Conversion of cacao pod husks by pyrolysis and catalytic reaction to produce useful chemicals. Biomass Bioenergy 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Dias, D.R.; Schwan, R.F.; Freire, E.S.; Dos Santos Serôdio, R. Elaboration of a fruit wine from cocoa (Theobroma cacao L.) pulp. Int. J. Food Sci. Technol. 2007, 42, 319–329. [Google Scholar] [CrossRef]
- Arlorio, M.; Coisson, J.; Restani, P.; Martelli, A. Characterization of pectins and some secondary compounds from Theobroma cacao hulls. J. Food Sci. 2001, 66, 653–656. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Solieri, L.; Giudici, P. Vinegars of the World, 1st ed.; Springer: Milan, Italy, 2009; ISBN 978-88-470-0866-3. [Google Scholar]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J.; Bangoura, M.L.; Lagnika, C. Quantification of total polyphenolic content and antimicrobial activity of cocoa (Theobroma cacao L.) bean shells. Pak. J. Nutr. 2012, 11, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran Nair, K.P. The Agronomy and Economy of Important Tree Crops of the Developing World, 1st ed.; Elsevier: London, UK, 2010; ISBN 978-0123846778. [Google Scholar]
- Yapo, B.M.; Besson, V.; Koubala, B.B.; Koffi, K.L. Adding value to cacao pod husks as a potential antioxidant-dietary fiber source. Am. J. Food Nutr. 2013, 1, 38–46. [Google Scholar] [CrossRef]
- Lessa, O.A.; dos Santos Reis, N.; Leite, S.G.F.; Gutarra, M.L.E.; Souza, A.O.; Gualberto, S.A.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Effect of the solid state fermentation of cocoa shell on the secondary metabolites, antioxidant activity, and fatty acids. Food Sci. Biotechnol. 2018, 27, 107–113. [Google Scholar] [CrossRef]
- Rahim, I.; Kuswinanti, T.; Asrul, L.; Rasyid, B. Screening of fungal rot isolates from cocoa as phosphate-dissolving and their growth ability on three types of media. Procedia Food Sci. 2015, 3, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Chocolate in Health and Nutrition; Humana Press: Heidelberg, Germany, 2013; ISBN 978-1-61779-802-3. [Google Scholar]
- Guest, D. Black pod: Diverse pathogens with a global impact on cocoa yield. Phytopathology 2007, 97, 1650–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endraiyani, V.; Ludescher, R.D.; Di, R.; Karwe, M.V. Total phenolics and antioxidant capacity of cocoa pulp: Processing and storage study. J. Food Process. Preserv. J. 2017, 41, e13029. [Google Scholar] [CrossRef]
- Dwapanyin, A.O.; Adomako, D.; Tetteh, J.P. The sugar content of cocoa sweatings and the effect of pressing the sweatings prior to fermentation on bean quality. J. Biochem. Mol. Biol. 1991, 1, 109–120. [Google Scholar]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Takrama, J.F.; Kumi, W.O.; Otoo, G.; Addo, K.; Camu, N. Optimization of cocoa pulp juice fermentation with yeast starter cultures of cocoa heap fermentations. J. Agric. Sci. Food Technol. 2015, 1, 22–33. [Google Scholar]
- Puerari, C.; Magalhães, K.T.; Schwan, R.F. New cocoa pulp-based kefir beverages: Microbiological, chemical composition and sensory analysis. Food Res. Int. 2012, 48, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Escalante, M.; Badrie, N.; Bekele, F.L. Production and quality characterization of pulp from cocoa beans from Trinidad: Effects of varying levels of pulp on value-added carbonated cocoa beverages. In Proceedings of the 49th Annual Meeting, Port of Spain, Trinidad and Tobago, 30 June–6 July 2013; Caribbean Food Crops Society: San Juan, Puerto Rico, 2013. ISSN 95-07-0410. [Google Scholar]
- Afolabi, M.O.; Ibitoye, W.O.; Agbaje, A.F. Evaluation of nutritional and sensory properties of cocoa pulp beverage supplemented with pineapple juice. J. Food Res. 2015, 4, 58–61. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Recobert, M.; Trautwein, G.; Pérez-Cadenas, M.; Alcañiz-Monge, J. Preparation of binderless activated carbon monoliths from cocoa bean husk. Microporous Mesoporous Mater. 2017, 243, 28–38. [Google Scholar] [CrossRef]
- Fioresi, F.; Vieillard, J.; Bargougui, R.; Bouazizi, N.; Fotsing, P.N.; Woumfo, E.D.; Brun, N.; Mofaddel, N.; Le Derf, F. Chemical modification of the cocoa shell surface using diazonium salts. J. Colloid Interface Sci. 2017, 494, 92–97. [Google Scholar] [CrossRef]
- DeVries, J.W. On defining dietary fibre. Proc. Nutr. Soc. 2003, 62, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kris-Etherton, P.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71S–88S. [Google Scholar] [CrossRef]
- Bessesen, D.H. The Role of Carbohydrates in Insulin Resistance. J. Nutr. 2001, 131, 2782S–2786S. [Google Scholar] [CrossRef]
- Johnson, I.T. New approaches to the role of diet in the prevention of cancers of the alimentary tract. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 551, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Valencia, N.; Granados-Pérez, E.; Agama-Acevedo, E.; Tovar, J.; Ruales, J.; Bello-Pérez, L.A. Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT Food Sci. Technol. 2007, 40, 722–729. [Google Scholar] [CrossRef]
- Do Espírito Santo, A.P.; Cartolano, N.S.; Silva, T.F.; Soares, F.A.S.M.; Gioielli, L.A.; Perego, P.; Converti, A.; Oliveira, M.N. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int. J. Food Microb. 2012, 154, 135–144. [Google Scholar] [CrossRef]
- Awang, A.; Karim, R.; Mitsui, T. Proteomic analysis of Theobroma cacao pod husk. J. App. Glycosci. 2010, 57, 245–264. [Google Scholar] [CrossRef] [Green Version]
- Martín-Cabrejas, M.A.; Valiente, C.; Esteban, R.M.; Mollá, E.; Waldron, K. Cocoa hull: A potential source of dietary fibre. J. Sci. Food Agric. 1994, 66, 307–311. [Google Scholar] [CrossRef]
- Minifie, B.W. Chocolate, Cocoa, and Confectionery: Science and Technology, 3rd ed.; Van Nostrand Reinhold: New York, NY, USA, 1989; ISBN 978-94-011-7926-3. [Google Scholar]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Agus, B.A.P.; Mohamad, N.N.; Hussain, N. Composition of unfermented, unroasted, roasted cocoa beans and cocoa shells from Peninsular Malaysia. J. Food Meas. Charact. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Donkoh, A.; Atuahene, C.C.; Wilson, B.N.; Adomako, D. Chemical composition of cocoa pod husk and its effect on growth and food efficiency in broiler chicks. Anim. Feed Sci. Technol. 1991, 35, 161–169. [Google Scholar] [CrossRef]
- Pätzold, R.; Brückner, H. Gas chromatographic determination and mechanism of formation of D-amino acids occurring in fermented and roasted cocoa beans, cocoa powder, chocolate and cocoa shell. Amino Acids 2006, 31, 63. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- El-Saied, H.M.; Morsi, M.K.; Amer, M.M.A. Composition of cocoa shell fat as related to cocoa butter. Z. Ernähr. 1981, 20, 145–151. [Google Scholar] [CrossRef]
- MolView. Available online: https://molview.org/ (accessed on 15 February 2022).
- Howlett, J.F.; Betteridge, V.A.; Champ, M.; Craig, S.A.S.; Meheust, A.; Jones, J.M. The definition of dietary fiber e discussions at the Ninth Vahouny Fiber Symposium: Building scientific agreement. Food Nutr. Res. 2010, 54, 112–129. [Google Scholar] [CrossRef]
- Peerajit, P.; Chiewchan, N.; Devahastin, S. Effects of pretreatment methods on health-related functional properties of high dietary fibre powder from lime residues. Food Chem. 2012, 132, 1891–1898. [Google Scholar] [CrossRef]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Deroanne, C.; Drira, N.E.; Attia, H. Date flesh: Chemical composition and characteristics of the dietary fibre. Food Chem. 2008, 111, 676–682. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Functional properties and characterization of dietary fiber from Mangifera pajang kort. fruit pulp. J. Agric. Food Chem. 2011, 59, 3980–3985. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Benería, M.A. Composition of dietary fibre in cocoa husk. Z. Lebensm. Unters Forsch. 1998, 207, 105–109. [Google Scholar] [CrossRef]
- Chung, B.Y.; Iiyama, K.; Han, K.W. Compositional Characterization of Cacao (Theobroma cacao L.) Hull. J. Appl. Biol. Chem. 2003, 46, 12–16. [Google Scholar]
- Redgwell, R.; Trovato, V.; Merinat, S.; Curti, D.; Hediger, S.; Manez, A. Dietary fibre in cocoa shell: Characterisation of component polysaccharides. Food Chem. 2003, 81, 103–112. [Google Scholar] [CrossRef]
- Abarca, D.; Martínez, R.; Muñoz, J.J.; Torres, M.P.; Vargas, G. Residuos de café, cacao y cladodio de tuna: Fuentes promisorias de fibra dietaria. Rev. Tecnol.-Espol 2010, 23, 63–69. [Google Scholar]
- Nazaruddin, R. Effect of ammonium oxalate and acetic acid at several extraction time and pH on some physicochemical properties of pectin from cocoa husks (Theobroma cacao). Afr. J. Food Sci. 2011, 5, 790–798. [Google Scholar] [CrossRef]
- Mollea, C.; Chiampo, F. Valorization of Cocoa Husks: Pectin Recovery. Int. J. Food Sci. 2019, 2019, 1212081. [Google Scholar] [CrossRef] [Green Version]
- Schwan, R.F.; Fleet, G.H. Cocoa and Coffee Fermentations, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1439847916. [Google Scholar]
- Martínez-Ángel, J.D.; Villamizar-Gallardo, R.A.; Ortíz-Rodríguez, O.O. Characterization and evaluation of cocoa (Theobroma cacao L.) pod husk as a renewable energy source. Agrociencia 2015, 49, 329–345. [Google Scholar]
- Sobamiwa, O.; Longe, O.G. Utilization of cocoa pod pericarp fractions in broiler chick diets. Animal Feed Sci. Technol. 1994, 47, 237–244. [Google Scholar] [CrossRef]
- Vītola, V.; Ciproviča, I. The effect of cocoa beans heavy and trace elements on safety and stability of confectionery products. Rural Sustain. Res. 2016, 35, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Bentil, J.A. Enhancement of the nutritive value of cocoa (Theobroma cacao) bean shells for use as feed for animals through a two-stage solid state fermentation with Pleurotus ostreatus and Aspergillus niger. Int. J. Appl. Microbiol. Biotechnol. Res. 2012, 3, 20–30. [Google Scholar]
- Thyssen, G.M.; Keil, C.; Wolff, M.; Sperling, M.; Kadow, D.; Haase, H.; Karst, U. Bioimaging of the elemental distribution in cocoa beans by means of LA-ICP-TQMS. J. Anal. At. Spectrom. 2018, 33, 187–194. [Google Scholar] [CrossRef]
- Mandrile, L.; Barbosa-Pereira, L.; Sorensen, K.M.; Giovannozzi, A.M.; Zeppa, G.; Engelsen, S.B.; Rossi, A.M. Authentication of cocoa bean shells by near-and mid-infrared spectroscopy and inductively coupled plasma-optical emission spectroscopy. Food Chem. 2019, 292, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Wickramasuriya, A.M.; Dunwell, J.M. Cacao biotechnology: Current status and future prospects. Plant Biotechnol. J. 2018, 16, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Knapp, A.W.; Coward, K.H. The vitamin D activity of cacao shell: The effect of the fermenting and drying of cacao on the vitamin D potency of cacao shell. II. The origin of vitamin D in cacao shell. Biochem. J. 1935, 29, 2728. [Google Scholar] [CrossRef] [PubMed]
- Kon, S.K.; Henry, K.M. The effect of feeding cacao shell to cows on the vitamin D content of butter (milk). Biochem. J. 1935, 29, 2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslam, E. Practical Polyphenolics: From Structure to Molecular Recognition and Physiological Action, 1st ed.; Cambridge University Press: New York, NY, USA, 2005; ISBN 9780521675598. [Google Scholar]
- Karim, A.A.; Azlan, A.; Ismail, A.; Hashim, P.; Gani, S.S.A.; Zainudin, B.H.; Abdullah, N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern. Med. 2014, 14, 381. [Google Scholar] [CrossRef] [Green Version]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos-Reyes, G.; Mendiola, J.A.; Ibáñez, E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- Tomas-Barberán, F.A.; Cienfuegos-Jovellanos, E.; Marín, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef]
- Hannum, S.M.; Erdman, J.W. Emerging health benefits from cocoa and chocolate. J. Med. Food 2000, 3, 73–75. [Google Scholar] [CrossRef]
- Forsyth, W.G.C.; Quesnel, V.C.; Roberts, J.B. The interaction of polyphenols and proteins during cacao curing. J. Sci. Food Agric. 1958, 9, 181–184. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Testa, G.; Sottero, B.; Poli, G.; Zeppa, G.; Biasi, F. A Dietary Mixture of Oxysterols Induces In Vitro Intestinal Inflammation through TLR2/4 Activation: The Protective Effect of Cocoa Bean Shells. Antioxidants 2019, 8, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation between Macrophages and Adipocytes In Vitro. Mol. Nutr. Food Res. 2019, 63, 1801413. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G. Effects of particle size and extraction methods on cocoa bean shell functional beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef] [Green Version]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Analytical dataset on volatile compounds of cocoa bean shells from different cultivars and geographical origins. Data Br. 2019, 25, 104268. [Google Scholar] [CrossRef]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Pavlović, N.; Jakovljević, M.; Miškulin, M.; Molnar, M.; Ačkar, Đ.; Jokić, S. Green extraction techniques of bioactive components from cocoa shell. Croat. J. Food Sci. Technol. 2019, 11, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Prior, M.Á.; Fernández-Bolaños, J.; de la Paz Aguilera-Herrera, M.; Rodríguez-Gutiérrez, G. Extra virgin olive oil jam enriched with cocoa bean husk extract rich in theobromine and phenols. LWT 2019, 111, 278–283. [Google Scholar] [CrossRef]
- Barišić, V.; Flanjak, I.; Križić, I.; Jozinović, A.; Šubarić, D.; Babić, J.; Miličević, B.; Ačkar, Đ. Impact of high-voltage electric discharge treatment on cocoa shell phenolic components and methylxanthines. J. Food Process Eng. 2019, 43, e13057. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Toda, T.A.; Oliveira, A.L.; Rodrigues, C.E.C. Effect of the temperature on the kinetics of cocoa bean shell fat extraction using pressurized ethanol and evaluation of the lipid fraction and defatted meal. Ind. Crops Prod. 2019, 130, 96–103. [Google Scholar] [CrossRef]
- Yusof, M.; Huzaimi, A.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E.; Zainudin, B.H. Optimization of an Ultrasound-Assisted Extraction Condition for Flavonoid Compounds from Cocoa Shells (Theobroma cacao) Using Response Surface Methodology. Molecules 2019, 24, 711. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.-L.; Muhammad, N.; Gu, Y.-C.; Yan, W.-D. A simple and efficient method for enrichment of cocoa polyphenols from cocoa bean husks with macroporous resins following a scale-up separation. J. Food Eng. 2019, 243, 82–88. [Google Scholar] [CrossRef]
- Utami, R.; Armunanto, R.; Supriyanto, S. Effects of cocoa bean (Theobroma cacao L.) fermentation on phenolic content, antioxidant activity and functional group of cocoa bean shell. Pak. J. Nutr. 2016, 15, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Lopes, A.S. The microbiota diversity identified during the cocoa fermentation and the benefits of the starter cultures use: An overview. Int. J. Food Sci. Technol. 2020, 56, 544–552. [Google Scholar] [CrossRef]
- Santos, R.X.; Oliveira, D.A.; Sodré, G.A.; Gosmann, G.; Brendel, M.; Pungartnik, C. Antimicrobial activity of fermented theobrama cacao pod husk extract. Gen. Mol. Res. 2014, 13, 7725–7735. [Google Scholar] [CrossRef]
- Díaz-Muñoz, C.; Van de Voorde, D.; Comasio, A.; Verce, M.; Hernandez, C.E.; Weckx, S.; De Vuyst, L. Curing of cocoa beans: Fine-scale monitoring of the starter cultures applied and metabolomics of the fermentation and drying steps. Front. Microbiol. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; Neto, D.C.N.; Gilberto, V.M.P.; Vandenberghe, L.P.S.; Oliveira, P.Z.; Tiburcio, P.B.; Tiburcio, H.L.G.; Neto, A.G.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Balladares, C.; Chóez Guaranda, I.; García, J.; Sosa, D.; Pérez, S.; González, J.E.; Viteri, R.; Barragán, A.; Quijano Avilés, M.; Manzano, P. Physicochemical characterization of Theobroma cacao L. mucilage, in Ecuadorian coast. Emir. J. Food Agric. 2016, 28, 741–745. [Google Scholar] [CrossRef]
- Neto, M.A.B.; Bonomo, R.C.F.; Fontan, R.C.I.; Ferreira, A.C.R.; Gonçalves, G.R.F.; Mello, D.L.N. Physical chemical characterization and thermophysical properties of cocoa honey. Rev. Gest. Inov. Tecnol. 2016, 6, 2944–2953. [Google Scholar] [CrossRef]
- Leite, P.B.; Machado, W.M.; Guimarães, A.G.; Carvalho, G.B.M.D.; Teixeira Magalhães-Guedes, K.; Izabel Druzian, J. Cocoa’s residual honey: Physicochemical characterization and potential as a fermentative substrate by Saccharomyces cerevisiae AWRI726. Sci. World J. 2019, 2019, 5698089. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2020, 1, 742–755. [Google Scholar] [CrossRef] [Green Version]
- Santana, D.P.; Sanchez, J.L.R.; Calle, J.; de Villavicencio, M.N.; Ortega, L.D.; Llanes, L.H. Utilización de la cascarilla de cacao como fuente de fibra dietética y antioxidantes en la elaboración de galletas dulces/Use of cocoa bean shell as a source of dietetic fiber and antioxidants in the production of sweet cookies. Cienc. Tecnol. Aliment. 2018, 28, 62–67. [Google Scholar]
- Collar, C.; Rosell, C.M.; Muguerza, B.; Moulay, L. Breadmaking performance and keeping behavior of cocoa-soluble fiber-enriched wheat breads. Food Sci. Technol. Int. 2009, 15, 79–87. [Google Scholar] [CrossRef]
- Öztürk, E.; Ova, G. Evaluation of cocoa bean hulls as a fat replacer on functional cake production. Turk. J. Agric.-Food Sci. Technol. 2018, 6, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cervera, S.; Salvador, A.; Muguerza, B.; Moulay, L.; Fiszman, S. Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT-Food Sci. Technol. 2011, 44, 729–736. [Google Scholar] [CrossRef]
- Ismail, A.; Yee, C.L. Antioxidative effects of extracts of cocoa shell, roselle seeds and a combination of both extracts on the susceptibility of cooked beef to lipid oxidation. J. Food Technol. 2006, 4, 10–15. Available online: https://medwelljournals.com/abstract/?doi=jftech.2006.10.15 (accessed on 15 January 2022).
- Manzano, P.; Hernández, J.; Quijano-Avilés, M.; Barragán, A.; Chóez-Guaranda, I.; Viteri, R.; Valle, O. Polyphenols extracted from Theobroma cacao waste and its utility as antioxidant. Emir. J. Food Agric. 2017, 29, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Osundahunsi, O.; Bolade, M.; Akinbinu, A. Effect of cocoa shell ash as an alkalizing agent on cocoa products. J. Appl. Sci. 2007, 7, 1674–1678. [Google Scholar] [CrossRef]
- Tran, T.N.; Heredia-Guerrero, J.A.; Mai, B.T.; Ceseracciu, L.; Marini, L.; Athanassiou, A.; Bayer, I.S. Bioelastomers based on cocoa shell waste with antioxidant ability. Adv. Sustain. Syst. 2017, 1, 1700002. [Google Scholar] [CrossRef]
- Syamsiro, M.; Saptoadi, H.; Tambunan, B.H.; Pambudi, N.A. A preliminary study on use of cocoa pod husk as a renewable source of energy in Indonesia. Energy Sustain. Dev. 2012, 16, 74–77. [Google Scholar] [CrossRef]
- Khanahmadi, S.; Yusof, F.; Amid, A.; Mahmod, S.S.; Mahat, M.K. Optimized preparation and characterization of CLEA-lipase from cocoa pod husk. J. Biotechnol. 2015, 202, 153–161. [Google Scholar] [CrossRef]
- Alemawor, F.; Dzogbefia, V.P.; Oddoye, E.O.; Oldham, J.H. Effect of Pleurotus ostreatus fermentation on cocoa pod husk composition: Influence of fermentation fermentation period and Mn2+ supplementation on the fermentation process. Afr. J. Biotechnol. 2009, 8, 1950–1958. [Google Scholar]
- Balentić, J.P.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, E.J.; Dilworth, B.C. Toxicity of jimson weed seed and cocoa shell meal to broilers. Poult. Sci. 1984, 63, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Magistrelli, D.; Zanchi, R.; Malagutti, L.; Galassi, G.; Canzi, E.; Rosi, F. Effects of Cocoa Husk Feeding on the Composition of Swine Intestinal Microbiota. J. Agric. Food Chem. 2016, 64, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Ayinde, O.E.; Ojo, V.; Adeyina, A.A.; Adesoye, O. Economics of Using Cocoa Bean Shell as Feed Supplement for Rabbits. Pak. J. Nutr. 2010, 9, 195–197. [Google Scholar] [CrossRef] [Green Version]
- Emiola, I.; Ojebiyi, O.; Akande, T. Performance and organ weights of laying hens fed diets containing graded levels of sun-dried cocoa bean shell (CBS). Int. J. Poult. Sci. 2011, 10, 986–989. [Google Scholar] [CrossRef] [Green Version]
- Meunier, N.; Laroulandie, J.; Blais, J.F.; Tyagi, R.D. Cocoa shells for heavy metal removal from acidic solutions. Bioresour. Technol. 2003, 90, 255–263. [Google Scholar] [CrossRef]
- Bello, O.S.; Ahmad, M.A. Adsorptive removal of a synthetic textile dye using cocoa pod husks. Toxicol. Environ. Chem. 2011, 93, 1298–1308. [Google Scholar] [CrossRef]
- Pua, F.L.; Sajab, M.S.; Chia, C.H.; Zakaria, S.; Rahman, I.A.; Salit, M.S. Alkaline-treated cocoa pod husk as adsorbent for removing methylene blue from aqueous solutions. J. Environ. Chem. Eng. 2013, 1, 460–465. [Google Scholar] [CrossRef]
- Krishna, C.P. A Research on Cocoa Pod Husk Activated Carbon for Textile Industrial Wastewater Colour Removal. Int. J. Res. Eng. Technol. 2014, 3, 731–737. [Google Scholar]
- Awolu, O.O.; Oyeyemi, S.O. Optimization of bioethanol production from cocoa (Theobroma cacao) bean shell. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 506–514. [Google Scholar]
- Ohguchi, K.; Tanaka, T.; Iliya, I.; Ito, T.; Iinuma, M.; Matsumoto, K.; Akao, Y.; Nozawa, Y. Gnetol as a potent tyrosinase inhibitor from genus Gnetum. Biosci. Biotechnol. Biochem. 2003, 67, 663–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Koiteh, Z. Conditioning Cleansing Cream. US Patent 8449895 B1, 28 May 2013. [Google Scholar]
- Abdul Karim, A.; Azlan, A.; Ismail, A.; Hashim, P.; Abd Gani, S.S.; Zainudin, B.H.; Abdullah, N.A. Efficacy of cocoa pod extract as antiwrinkle gel on human skin surface. J. Cosmet. Dermat. 2016, 15, 283–295. [Google Scholar] [CrossRef]
- Martin, M.Á.; Goya, L.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Compounds | Cocoa Husk | Cocoa Pulp | Cocoa Bean Shell | References |
|---|---|---|---|---|
| Carbohydrates | 29.04–32.30 | 10.70–68.35 | 17.80–23.17 | [18,19] |
| Cellulose | 24.24–35.00 | 20.80–57.50 | 15.10 | [20] |
| Hemicellulose | 8.72–11.00 | 7.00–17.00 | - | [21] |
| Lignin | 14.60–26.38 | 12.00-14.60 | 32.41 | [22] |
| Pectin | 6.10–9.20 | 0.57–1.50 | 0.57–1.50 | [23,24] |
| Total dietary fibre | 36.60–56.10 | 16.89 | 18.60–60.60 | [17,25] |
| Total proteins | 4.21–10.74 | 0.41–5.56 | 15.79–18.10 | [26,27] |
| Lipids | 1.50–2.24 | 1.91–3.54 | 2.02–6.87 | [18,28] |
| Ash | 6.70–10.02 | 3.70–7.68 | 5.96–11.42 | [21,27] |
| Minerals (mg/100 g) | 3230.85 | 1297.07 | 56.75–312.57 | [17] |
| Total organic acids | - | 17.52 | - | [18] |
| Total phenolics * | 4.60–6.90 | - | 1.32–5.78 | [29] |
| Anthocyanins ** | - | - | 0.40 | [30] |
| Theobromine | 0.34 | - | 1.30 | [20,24] |
| Caffeine | - | - | 0.10 | [28] |
| Tannins | 5.20 | - | 3.30–4.46 | [22,29] |
| Flavonols ** | - | - | 1.50 | [30] |
| Amino Acids | Cocoa Husk | Cocoa Bean Shell |
|---|---|---|
| Essential | 2.66 | 4.15 |
| Arginine | 0.22 | 0.70 |
| Histidine | 0.21 | 0.27 |
| Isoleucine | 0.24 | 0.48 |
| Leucine | 0.43 | 0.45 |
| Lysine | 0.40 | 0.79 |
| Methionine | 0.05 | 0.06 |
| Phenylalanine | 0.37 | 0.45 |
| Threonine | 0.30 | 0.70 |
| Valine | 0.44 | 0.25 |
| Non Essential | 3.43 | 6.59 |
| Aspartic acid | 0.80 | 1.50 |
| Alanine | 0.44 | 0.80 |
| Cystine | 0.09 | 0.25 |
| Glycine | 0.29 | 0.72 |
| Glutamic acid | 0.77 | 1.87 |
| Proline | 0.38 | 0.20 |
| Serine | 0.41 | 0.71 |
| Tryptophan | 0.04 | 0.12 |
| Tyrosine | 0.21 | 0.42 |
| Total amino acids | 6.09 | 10.74 |
| BCAA | 1.11 | 1.18 |
| AAA | 0.83 | 1.26 |
| Characteristics | Cocoa Butter | Cocoa Bean Shell |
|---|---|---|
| Specific gravity at 40 °C | 0.9012 | 0.9034 |
| Melting point (°C) | 34.10 | 31.00 |
| Acid value (expressed as oleic acid %) | 1.68 | 9.12 |
| Saponification index | 191.214 | 205.708 |
| Iodine index | 35.57 | 38.73 |
| Fatty Acid | Cocoa Butter | Cocoa Bean Shell | |
|---|---|---|---|
| Capric | (C 10:0) | 12.95 | 16.89 |
| Lauric | (C 12:0) | Traces | Traces |
| Tridecanoic | (C 13:0) | Traces | Traces |
| Myristic | (C 14:0) | 4.32 | 3.19 |
| Myristoleic | (C 14:1) | 1.29 | 2.43 |
| Palmitic | (C 16:0) | 23.31 | 22.27 |
| Palmitoleic | (C 16:1) | 0.95 | 2.55 |
| Margaric | (C 17:0) | Traces | Traces |
| Stearic | (C 18:0) | 24.51 | 12.05 |
| Oleic | (C 18:1) | 28.74 | 28.16 |
| Linoleic | (C 18:2) | 3.93 | 7.49 |
| By-Product | Total (g/100 g d.w.) | Soluble (g/100 g d.w.) | Insoluble (g/100 g d.w.) | Ratio Insol./Sol. |
|---|---|---|---|---|
| Cocoa pulp | 16.75–16.89 | 16.06–16.11 | 0.69–0.78 | 0.04–0.05 |
| Cocoa husk | 55.09–56.10 | 2.88–4.12 | 51.98–53.11 | 12.89–18.05 |
| Cocoa bean shell | 51.88–56.70 | 14.53–16.24 | 35.64–42.17 | 2.45–2.53 |
| Dietary Fibre Composition | (% Dry Weight) | |
|---|---|---|
| Soluble | Insoluble | |
| Neutral sugars a | 2.96 | 14.53 |
| Rhamnose | 0.29 | 0.15 |
| Fucose | Not detected | 0.06 |
| Arabinose | 0.29 | 0.94 |
| Xilose | 0.09 | 0.97 |
| Mannose | 0.51 | 0.96 |
| Galactose | 1.36 | 0.91 |
| Glucose | 0.41 | 10.53 |
| Uronic Acids | 7.13 | 3.48 |
| Klason Lignin | - | 32.41 |
| Total | 10.09 | 50.42 |
| Compound | Percentage (in Dry Matter) | |
|---|---|---|
| Redgwell et al. (2003) [65] | Lecumberri et al. (2007) [22] | |
| Total dietary fibre | 63.6 | 60.5 |
| Total polysaccharides | 38.2 | 28.1 |
| Minerals | Mineral Content (mg/100 g) | References | ||
|---|---|---|---|---|
| Cocoa Husk | Cocoa Pulp | Cocoa Bean Shell | ||
| Ca | 254.00 | 171.50 | 230.00–440.00 | [18,65] |
| Cu | 6.18 | - | 2.35–6.62 | [17,65] |
| Fe | 5.80 | - | 27.60–80.50 | [18,64] |
| K | 2768.00 | 950.00 | 1250.00–1820.00 | [18,65] |
| Mg | 100.90 | 82.50 | 480.00–1290.00 | [17,64] |
| Mn | 35.72 | - | 4.53 | [17,65] |
| Na | 10.60 | 30.50 | 16.00–192.20 | [18,64] |
| P | - | 62.47 | 580.00–1000.00 | [64,65] |
| Se | 0.01 | - | 0.21 | [17,65] |
| Zn | 39.74 | - | 2.75–19.00 | [17,73] |
| Compounds | Concentration (mg/g) |
|---|---|
| (−)-epicatechin | 0.21–34.97 |
| (+)-catechin | 0.18–4.50 |
| Epicatechin-(4β→8)-catechin | 0.55–0.83 |
| Epicatechin-(4β)→8)-epicatechin | 0.23–1.38 |
| Compounds | Concentration |
|---|---|
| Total phenolic compounds (mg GAE/g d.m.) | 6.04–94.95 |
| Total flavonoids (mg CE/g d.m.) | 1.65–40.72 |
| Total tannin (mg CE/g d.m.) | 1.70–25.30 |
| By-Product | Methodology | Results | References |
|---|---|---|---|
| Cocoa husk | Crushed and carbonized at 400 °C for a period of 2 h. | Generation of a higher heating value (17 MJ/kg) with high ash content. | [115] |
| Cocoa husk | Generation of a solid base catalyst for the transesterification of soy oil into biodiesel. | Potassium from cocoa husk can be a viable base catalyst generating high yields for biodiesel production, as well as better engine performance. | [20] |
| Cocoa husk | Lipase immobilization through crosslinking enzymatic aggregate technology. | The immobilized enzyme is a potential catalyst for the production of biodiesel by transesterification of Jatropha curcas oil. | [116] |
| Cocoa husk | Conversion of cocoa husk through a pyrolysis process and catalytic reactions. | Production of useful chemicals such as ketones, carboxylic acids, aldehydes, furans, heterocyclic aromatics, alkylbenzenes, phenols and benzenediols. | [21] |
| Cocoa husk | Use and optimization of a fermentation process with the mushroom Pleurotus ostreatus as a biocatalyst. | After five weeks of fermentation with 0.075% (w/w) MnCl2, 36% increase in crude protein and total soluble carbohydrates was generated with a 17% reduction in fibre and 88% in total tannins. | [117] |
| Cocoa bean shell | Adsorption and desorption of phosphate-P, ammonium-N and nitrate-N in corncob biochars. | Biochar can release essential nutrients to the soil to improve being able to release PO43−–P and weakly exchange NH4+–N. | [118] |
| Cocoa bean shell | Addition of the shell or theobromine, in different concentrations, ranging from 1, 2, 4 and 6% to the chicken feed. | In the proportions of 4 and 6% of husk there was a significant influence on the decrease in body weight of chickens and for theobromine the weight of chickens was drastically reduced. | [119] |
| Cocoa bean shell | Six pigs were fed a conventional cereal-based diet, or a diet obtained by substitution of 7.5% of the conventional diet with cocoa shell for 3 weeks. | An increase in microbial populations of the Bacteroides-Prevotella and Faecalibacterium prausnitzii group and a reduction in Lactobacilli, however, this feeding improved the proportion between the main phyla of the intestinal ecosystem. | [120] |
| Cocoa bean shell | This study was collected from an experimental study of performance of rabbits fed graded levels of various treatments of shell as feed supplement. | It is concluded that untreated cocoa bean shell can be used in the inclusion of 100 g/kg in the rabbit feed, while those treated with hot water can be included up to 200 g/kg in the rabbit feed for growth performance with ideal and the highest cost-benefit ratio. | [121] |
| Cocoa bean shell | Assessment of increased intake of sun-dried shell, with a concentration between 0 to 30%. | Reduction in average daily feed intake and egg production, as well as in spleen, kidney and ovary weight in chickens fed 25 and 30% concentration feeds, due to increased theobromine intake. | [122] |
| By-Product | Methodology | Results | References |
|---|---|---|---|
| Cocoa husk | Adsorption tests were performed under agitation with different metallic elements and cocoa husk concentrations. | Efficient in removing lead from acidic solutions, with maximum adsorption after 2 h. It was also observed that the other metals do not influence lead adsorption in the matrix. | [123] |
| Cocoa husk | The cocoa husk (1–2 mm size) was activated with the reactive orange dye and subsequently carbonized between 500 °C and 700 °C. | The kinetics showed that the material is an effective adsorption agent with a maximum adsorption of 111 mg/g of Remazol Brilliant Black R, for its use as a dye removing agent in textile effluents. | [124] |
| Cocoa husk | The cocoa husk underwent an alkaline treatment (NaOH) for the adsorption of methylene blue. | The maximum adsorption capacity of methylene blue is 263.9 mg/g, where a pseudo-second order provides the best correlation to predict the kinetic process. The adsorption of methylene blue was considered endothermic and spontaneous. | [125] |
| Cocoa husk | Cocoa husks were used as a precursor to the activated carbon for dye removal from textile industry effluents. | The best results obtained were from the production of activated charcoal with cocoa husk, being chemically activated with ZnCl2 and subsequently carbonized. Removal levels reached about 80% in a period of less than 1 h with pore sizes of 0.25–1 mm. | [126] |
| Cocoa bean shell | Ethanol production from cocoa bean shell using acid hydrolysis and Saccharomyces cerevisiae. | The pH has the most relevant effect on the yield of ethanol production, followed by the fermentation time and, finally, the yeast concentration. Cocoa bean shells and the developed methodology are excellent for an optimization of ethanol production. | [127] |
| Cocoa bean shell | Energy use evaluation of solid biofuels (wheat straw and rapeseed) and their mixtures with suitable additives (cocoa bean shell, lignite and coal sludge). | The results of thermal emission measurements demonstrated that all samples meet the requirements for carbon monoxide, but the average emission concentrations of nitrogen oxides exceed the limits. | [118] |
| By-Product | Field | Methodology | Results | References |
|---|---|---|---|---|
| Cocoa husk | Cosmetics | Extraction of cocoa husk with an ethanolic solvent (80%) to study the effect of skin lightening | A sun protection effect was observed from the in vitro mushroom tyrosinase assay (in the absorption range between 200–400 nm wavelength). | [82] |
| Cocoa husk | Cosmetics | Resveratrol and fatty acids, such as linoleic acid, were isolated from an acetone-soluble extract of cocoa husk. | In the results obtained, it was observed that such compounds have in vitro skin lightening properties and do not cause adverse effects. | [128,129] |
| Cocoa husk | Cosmetics | To make African Black soap, cocoa husk ash is used, along with Cocos nucifera (coconut oil), Butyrospermum parkii (raw shea butter), among others, and water. | Soap is used in environmentally friendly cleansers and conditioners. | [130] |
| Cocoa husk | Cosmetics | For the determination of EC50, fibroblast cells were used. Finally, the gel was tested by 12 panel members to determine the effectiveness of cocoa husk extracts in gel form using Visioscan to reduce skin wrinkles and improve skin condition. | From the results it was observed that the extract is a potential ingredient for wrinkle reduction. In which the wrinkles of the skin reduced around between 6 to 13%, between 3 and 5 weeks, still generating an increase in the hydration of the skin, around 3% after 3 weeks of application of the gel. | [131] |
| Cocoa husk | Antibacterial | To generate the crude extract, the cocoa husk underwent a spontaneous aerobic fermentation process. The generated extract was fractionated by solvent partition with polar solvent extraction or by silica gel chromatography, in which they were analysed for chemical composition and bioactivity. | The extract showed efficacy against Gram-negative Salmonella choleraesuis (ATCC10708) (1 mg/mL MIC) and Gram-positive Staphylococcus epidermidis (ATCC35984) (2.5 mg/mL MIC), still showing a high inhibitory activity against Pseudomonas aeruginosa (ATCC15442). | [100] |
| Cocoa bean shell | Cardiovascular diseases | Investigation of cocoa flavonols due to their antioxidant activity in plasma, causing a decrease in platelet reactivity or their anti-inflammatory properties. | It was observed that such compounds are correlated with the prevention of some diseases, such as cardiovascular diseases, due to their properties being correlated with the reduction of the potential for the emergence of atherosclerosis or thrombosis. | [45] |
| Cocoa bean shell | Cardiovascular diseases | Investigation of the in vivo bioavailability of cocoa bean shell from dietary intake and its contribution to cardiovascular health. | It was observed that cocoa bean shell fibre has a considerable ability to adsorb a large amount of oil and cholesterol, thus reducing its bioavailability during the digestion process. | [27] |
| Cocoa bean shell | Diabetes and obesity | In vivo studies were carried out, with the help of rats, to verify changes in lipid and cholesterol rates, from the ingestion of cocoa bean shell. | Significant reductions in total cholesterol and low-density lipoprotein were observed, due to the effect resulting mainly from the soluble part of dietary fibre. | [22] |
| Cocoa bean shell | Diabetes | The studies were carried out in vitro simulating a diabetic condition in different cell lines from or obtained from the main target tissues for the disease, to verify the efficiency of flavanols. | Cocoa flavonols act as chemo-preventive agents, helping to prevent or treat type 2 diabetes mellitus, as they regulate insulin secretion and protect pancreatic-β cells, in which they still have insulin-like activity, helping to improve glucose transport. for some organs. | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, T.F.; Oliveira, M.B.P.P. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. https://doi.org/10.3390/molecules27051625
Soares TF, Oliveira MBPP. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules. 2022; 27(5):1625. https://doi.org/10.3390/molecules27051625
Chicago/Turabian StyleSoares, Thiago F., and M. Beatriz P. P. Oliveira. 2022. "Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects" Molecules 27, no. 5: 1625. https://doi.org/10.3390/molecules27051625