Elsholtzia ciliata (Thunb.) Hyl. Extracts from Different Plant Parts: Phenolic Composition, Antioxidant, and Anti-Inflammatory Activities
Abstract
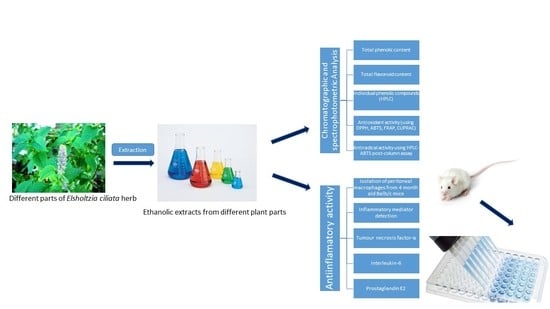
:1. Introduction
2. Results and Discussion
2.1. Determination of Total Phenolic Content
2.2. Determination of Total Flavonoid Content
2.3. Measurements of Antioxidant Activity in Extracts
2.4. Identification and Quantification of Phenolic Compounds by High-Performance Liquid Chromatography (HPLC)
2.5. Analysis of the Antiradical Activity of E. ciliata Ethanolic Extracts Using HPLC-ABTS Post-Column Assay
2.6. Anti-Inflammatory Activity of the Ethanolic Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Preparation of E. ciliata Ethanolic Extracts
3.4. Determination of Total Phenolic Content
3.5. Determination of Total Flavonoid Content
3.6. Determination of Antioxidant Activity
3.6.1. DPPH·Scavenging Assay
3.6.2. ABTS·+ Decolorization Assay
3.6.3. FRAP Assay
3.6.4. CUPRAC Assay
3.6.5. Calculation of Antioxidant Activity of the E. ciliata Ethanolic Extracts
3.7. HPLC Analysis, ABTS Post-Column Assay
3.8. Cell Culture and Treatments
3.9. Viability Staining
3.10. Inflammatory Mediator Detection
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-salam, E.M.; Qahtan, A.A.; Cantini, C. Production of Plant Secondary Metabolites: Examples, Tips, and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food System: A Mini-Review. Front. Nutr. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, A.; DellaGreca, M.; D’Abrosca, B.; Oriano, P.; Golino, A.; Izzo, A.; Zarrelli, A.; Monaco, P. Lignans, neolignans and sesquilignans from Cestrum parqui l’Her. Biochem. Syst. Ecol. 2007, 35, 392–396. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, R.; Terry, L.A. Berry antioxidants: small fruits providing large benefits. J. Sci. Food Agric. 2013, 94, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Mocanu, M.M.; Nagy, P.; Szöllosi, J. Chemoprevention of breast cancer by dietary polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability, and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Food Sci. Biotechnol. 2016, 80, 97–108. [Google Scholar]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Temussi, F.; Zarrelli, A. New dimeric phenanthrenoids from the rhizomes of Juncus acutus. Structure determination and antialgal activity. Tetrahedron 2003, 59, 2317–2324. [Google Scholar] [CrossRef]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Zarrelli, A. Low molecular weight phenols from the bioactive aqueous fraction of Cestrum parqui. J. Agric. Food Chem. 2004, 52, 4101–4108. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, M.S.; Niciforovic, N.; Mihailovic, V.; Topuzovic, M.; Solujic, S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp. polium. Acta Soc. Bot. Pol. 2012, 81, 117–122. [Google Scholar] [CrossRef]
- Sicari, V.; Pellicanò, T.M.; Giuffrè, A.M.; Zappia, C.; Capocasale, M.; Poiana, M. Physical chemical properties and antioxidant capacities of grapefruit juice (Citrus paradisi) extracted from two different varieties. Int. Food Res. J. 2018, 25, 1978–1984. [Google Scholar]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Yoo, J.S.; Lee, H.S.; Kwon, T.K.; Shin, T.Y.; Kim, S.H. Elsholtzia ciliata inhibits mast cell-mediated allergic inflammation: role of calcium, p38 mitogen-activated protein kinase and nuclear factor-{kappa}B. Exp. Biol. Med. 2011, 236, 1070–1077. [Google Scholar] [CrossRef]
- Petek, M.; Pintar, J.; Satovic, Z. Medicinal Plants of the Family Lamiaceae as Functional Foods—A Review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects, and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Zhao, P.M.; Liu, C.X.; Lai, D.; Zhou, L.; Liu, L.Z. Analysis of the essential oil of Elsholtzia ciliate aerial parts and its insecticidal activities against Liposcelis bostrychophila. Helv. Chim. Acta 2016, 99, 90–94. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Jiang, H. Study on the differences between volatile constituents of the different parts from Elsholtzia ciliata by SHS-GC-MS. Am. J. Analyt. Chem. 2017, 8, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.S.; Choi, B.; Kim, S.H.; Pak, S.C.; Jang, C.H.; Chin, Y.; Kim, Y.; Kim, D.; Jeon, S.; Koo, B. Essential oils from the medicinal herbs upregulate dopamine transporter in rat pheochromocytoma cells. J. Med. Food 2015, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, Z.; Wang, X.; Liu, W.; Jiang, R.; Cheng, R.; She, G. Elsholtzia: phytochemistry and biological activities. Chem. Cent. J. 2012, 6, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pudziuvelyte, L.; Stankevicius, M.; Maruska, A.; Petrikaite, V.; Ragazinskiene, O.; Draksiene, G.; Bernatoniene, J. Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind. Crops Prod. 2017, 107, 90–96. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Jakstas, V.; Ivanauskas, L.; Laukeviciene, A.; Ibe, D.F.C.; Kursvietiene, L.; Bernatoniene, J. Different extraction methods for phenolic and volatile compounds recovery from Elsholtzia ciliata fresh and dried herbal materials. Ind. Crops Prod. 2018, 120, 286–294. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Anal. Biochem. 2011, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from Northeast of Algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 760–766. [Google Scholar] [CrossRef]
- Yan, R.; Cao, Y.; Yang, B. HPLC-DPPH Screening Method for Evaluation of Antioxidant Compounds Extracted from Semen Oroxyli. Molecules 2014, 19, 4409–4417. [Google Scholar] [CrossRef] [Green Version]
- Ou, Z.; Schmierer, D.M.; Rades, T.; Larsen, L.; Mcdowell, A. Application of an online post-column derivatization HPLC-DPPH assay to detect compounds responsible for antioxidant activity in Sonchus oleraceus L. leaf extracts. J. Pharm. Pharmacol. 2013, 65, 271–279. [Google Scholar] [CrossRef]
- Liu, X.; Jia, J.; Yang, L.; Yang, F.; Ge, H.; Zhao, C.; Zhang, L.; Zu, Y. Evaluation of antioxidant activities of aqueous extracts and fractionation of different parts of Elsholtzia ciliate. Molecules 2012, 17, 5430–5441. [Google Scholar] [CrossRef]
- Juhaimi, A.F.; Ghafoor, K. Total phenols and antioxidant activities of leaf and stem extracts from coriander, mint, and parsley grown in Saudi Arabia. Pak. J. Bot. 2011, 43, 2235–2237. [Google Scholar]
- Sepúlveda-Jiménez, G.; Reyna-Aquino, C.; Chaires-Martínez, L.; Bermúdez-Torres, K.; Rodríguez-Monroy, M. Antioxidant activity, and content of phenolic compounds and flavonoids from Justicia spicigera. Pak. J. Biol. Sci. 2009, 9, 629–632. [Google Scholar] [CrossRef] [Green Version]
- Trpevski, M.; Lozanovska, I.; Talevska, A.; Ugurovska, D. Phenolic and Flavonoid Contents of Some Medicinal Plants from Jablanica Mt., Republic of Macedonia. In Proceedings of the III Congress of Ecologists of Macedonia, Struga, Macedonia, 6–9 October 2007. [Google Scholar]
- Liaudanskas, M.; Viškelis, P.; Raudonis, R.; Kviklys, D.; Uselis, N.; Janulis, V. Phenolic composition and antioxidant activity of Malus domestica leaves. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Advances 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.P.; Shi, S.Y.; Xiong, X.; Chen, X.Q.; Peng, M.J. Comparative evaluation of three methods based on high-performance liquid chromatography analysis combined with a 2,2′-diphenyl-1-picrylhydrazyl assay for the rapid screening of antioxidants from Pueraria lobata flower. Anal. Bioanal. Chem. 2012, 402, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Kim, Y.J.; Seo, C.S.; Kim, H.T.; Park, S.R.; Lee, M.Y.; Jung, J.Y. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-ß and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine 2016, 23, 331–339. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Dai, J.; Mumpr, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: chemistry, meta-bolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215–217. [Google Scholar] [CrossRef]
- Raudonis, R.; Raudone, L.; Jakstas, V.; Janulis, V. Comparative evaluation of post-column free radical scavenging and ferric reducing antioxidant power assays for screening of antioxidants in strawberries. J. Chromatogr. A 2012, 1233, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Verpoorte, R.; Korthout, H.A.A.J.; Mustafa, N.R. Phytochemicals as a potential source for TNF-α inhibitors. Phytochem. Rev. 2013, 12, 65–93. [Google Scholar] [CrossRef]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 2013, 110, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulrich-Merzenich, G.; Hartbrod, F.; Kelber, O.; Müller, J.; Koptina, A.; Zeitler, H. Salicylate-based phytopharmaceuticals induce adaptive cytokine and chemokine network responses in human fibroblast cultures. Phytomedicine 2017, 34, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-N.; Yan, E.-Z.; Wang, H.-M.; Sui, H.-J.; Liu, Z.; Gao, W.; Jin, Y. Hyperoside exerts anti-inflammatory and anti-arthritic effects in LPS-stimulated human fibroblast-like synoviocytes in vitro and in mice with collagen-induced arthritis. Acta Pharm. Sin. 2016, 37, 674–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, B.-R.; Chung, K.-S.; Cheon, S.-Y.; Lee, M.; Hwang, S.; Noh Hwang, S.; Rhee, K.-J.; An, H.-J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 7, 46252. [Google Scholar] [CrossRef]
- Wen, Y.-J.; Yin, M.-C. The anti-inflammatory and anti-glycative effects of rosmarinic acid in the livers of type 1 diabetic mice. Biomedicine 2017, 7, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Ambriz-Perez, D.L.; Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent. Food Agric. 2016, 2, 1–16. [Google Scholar]
- López-Posadas, R.; Ballester, I.; Mascaraque, C.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Flavonoids exert distinct modulatory actions on cyclooxygenase 2 and NF-kappaB in an intestinal epithelial cell line (IEC18). Br. J. Pharmacol. 2010, 160, 1714–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Vo, V.A.; Lee, J.-W.; Chang, J.-E.; Kim, J.-Y.; Kim, N.-H.; Lee, H.J.; Kim, S.-S.; Chun, W.; Kwon, Y.-S. Avicularin Inhibits Lipopolysaccharide-Induced Inflammatory Response by Suppressing ERK Phosphorylation in RAW 264.7 Macrophages. Biomol. Ther. 2012, 20, 532–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Dong, L.; Dang, X.; Liu, Y.; Jiang, J.; Wang, Y.; Lu, X.; Guo, X. Effect of chlorogenic acid on LPS-induced proinflammatory signaling in hepatic stellate cells. Inflamm. Res. 2013, 62, 581–587. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. Integr. Med. Res. 2015, 9, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Yim, S.H.; Nam, S.H. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1998, 299, 15–27. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E.; Erça, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Jakštas, V.; Raudonis, R.; Kviklys, D.; Milašius, A.; Janulis, V. Application of an optimized HPLC method for the detection of various phenolic compounds in apples from Lithuanian cultivars. J. Chem. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Lu, M.; Varley, A. Harvest and Culture of Mouse Peritoneal Macrophages. Bio-Protocol. 2013, 3, e976. [Google Scholar] [CrossRef]
Sample Availability: Samples of the E. ciliata ethanolic extracts are available from the authors. |









| Quercetin Glycosides | ||||
|---|---|---|---|---|
| Extracts | Rutin | Hyperoside | Quercitrin | Avicularin |
| Leaves | 0.076 ± 0.002 a | 0.017 ± 0.001 a | 0.088 ± 0.004 a | 0.230 ± 0.009 a |
| Stems | 0.090 ± 0.001 a | 0.017 ± 0.001 a | 0.048 ± 0.002 b | 0.027 ± 0.001 c |
| Whole plant | 0.992 ± 0.079 b | 0.126 ± 0.001 b | 0.088 ± 0.002 a | 0.294 ± 0.034 a |
| Flowers | 2.286 ± 0.230 c | 0.233 ± 0.026 c | 0.075 ± 0.004 a | 0.481 ± 0.212 b |
| Phenolic Acids | ||||
|---|---|---|---|---|
| Extracts | Chlorogenic Acid | Caffeic Acid | Rosmarinic Acid | p-Coumaric Acid |
| Leaves | 10.477 ± 0.391 a | 0.044 ± 0.0001 a | 0.115 ± 0.011 a | 0.068 ± 0.003 |
| Stems | 2.748 ± 0.051 b | 0.035 ± 0.0008 b | 0.412 ± 0.017 ac | ND |
| Whole plant | 14.910 ± 0.855 c | 0.041 ± 0.0001 c | 0.959 ± 0.066 bc | ND |
| Flowers | 3.023 ± 0.051 bd | 0.038 ± 0.00002 d | 1.347 ± 0.443 d | ND |
| Antioxidant Compound | RT (min) | Leaves Extract | Flowers Extract | Whole Herb Extract | Stems Extract |
|---|---|---|---|---|---|
| Unknown | 13.93 | 2.12 ± 0.1 | 0.534 ± 0.03 | 2.24 ± 0.07 | 0.51 ± 0.02 |
| Unknown | 15.70 | 0.314 ± 0.01 | 0.322 ± 0.03 | 0.36 ± 0.01 | 0.17 ± 0.01 |
| Unknown | 17.59 | 1.153 ± 0.15 | 1.04 ± 0.05 | 1.46 ± 0.05 | 0.28 ± 0.02 |
| Chlorogenic acid | 21.66 | 5.423 ± 0.13 | 16.38 ± 0.08 | 12.43 ± 0.1 | 4.04 ± 0.06 |
| Unknown | 22.30 | 4.535 ± 0.09 | 1.851 ± 0.09 | 5.48 ± 0.05 | 0.57 ± 0.02 |
| Caffeic acid | 23.60 | 0.153 ± 0.03 | 0.396 ± 0.03 | 0.28 ± 0.01 | 0.56 ± 0.03 |
| Unknown | 26.54 | 0.394 ± 0.02 | 0.495 ± 0.01 | 0.56 ± 0.02 | 0.43 ± 0.02 |
| Unknown | 29.31 | 3.01 ± 0.08 | 0.359 ± 0.03 | 3.07 ± 0.06 | 0.52 ± 0.04 |
| p-coumaric acid | 32.41 | 0.142 ± 0.01 | ND | ND | ND |
| Rutin | 40.40 | 0.064 ± 0.01 | 2.257 ± 0.05 | 0.81 ± 0.03 | 0.21 ± 0.02 |
| Hyperoside | 41.03 | 0.061 ± 0.01 | 0.599 ± 0.03 | 0.22 ± 0.01 | 0.14 ± 0.01 |
| Luteolin-7-O-glucoside | 41.80 | 0.132 ± 0.02 | 0.287 ± 0.03 | 0.20 ± 0.01 | 0.33 ± 0.03 |
| Avicularin | 43.95 | 0.268 ± 0.02 | 2.519 ± 0.06 | 1.74 ± 0.06 | 0.16 ± 0.01 |
| Apigenin-7-O-glucoside | 44.90 | 0.083 ± 0.01 | 0.358 ± 0.03 | 0.03 ± 0.001 | 0.06 ± 0.01 |
| Quercitrin | 45.25 | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.02 | 0.048 ± 0.0 |
| Rosmarinic acid | 47.02 | 0.501 ± 0.02 | 3.806 ± 0.02 | 3.32 ± 0.08 | 1.23 ± 0.06 |
| Unknown | 50.13 | 0.028 ± 0.01 | 0.061 ± 0.01 | 0.1 ± 0.01 | 0.62 ± 0.02 |
| Unknown | 52.15 | 0.07 ± 0.01 | 2.059 ± 0.06 | 1.38 ± 0.06 | 0.19 ± 0.02 |
| Apigenin | 53.70 | 0.045 ± 0.01 | 0.072 ± 0.01 | ND | 0.32 ± 0.03 |
| Diosmetin | 54.15 | ND | 0.038 ± 0.01 | 0.07 ± 0.01 | 0.11 ± 0.02 |
| Unknown | 57.04 | 0.74 ± 0.03 | 0.654 ± 0.01 | 0.9 ± 0.02 | 0.24 ± 0.01 |
| Total of all quantitated compounds | 19.37 ± 0.78 a | 34.20 ± 0.68 b | 34.77 ± 0.68 b | 10.74 ± 0.47 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudziuvelyte, L.; Liaudanskas, M.; Jekabsone, A.; Sadauskiene, I.; Bernatoniene, J. Elsholtzia ciliata (Thunb.) Hyl. Extracts from Different Plant Parts: Phenolic Composition, Antioxidant, and Anti-Inflammatory Activities. Molecules 2020, 25, 1153. https://doi.org/10.3390/molecules25051153
Pudziuvelyte L, Liaudanskas M, Jekabsone A, Sadauskiene I, Bernatoniene J. Elsholtzia ciliata (Thunb.) Hyl. Extracts from Different Plant Parts: Phenolic Composition, Antioxidant, and Anti-Inflammatory Activities. Molecules. 2020; 25(5):1153. https://doi.org/10.3390/molecules25051153
Chicago/Turabian StylePudziuvelyte, Lauryna, Mindaugas Liaudanskas, Aiste Jekabsone, Ilona Sadauskiene, and Jurga Bernatoniene. 2020. "Elsholtzia ciliata (Thunb.) Hyl. Extracts from Different Plant Parts: Phenolic Composition, Antioxidant, and Anti-Inflammatory Activities" Molecules 25, no. 5: 1153. https://doi.org/10.3390/molecules25051153