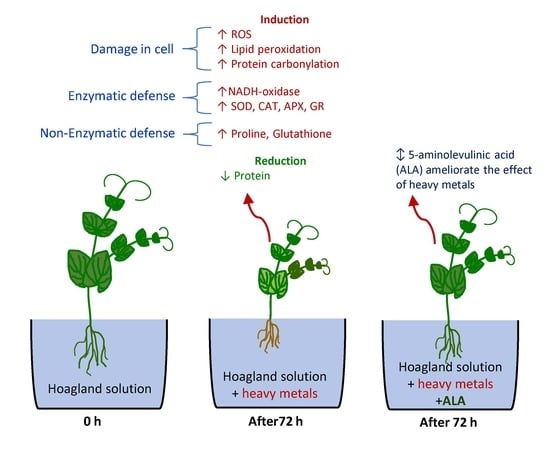
Antioxidant System and Biomolecules Alteration in Pisum sativum under Heavy Metal Stress and Possible Alleviation by 5-Aminolevulinic Acid
Abstract
:1. Introduction
2. Results
2.1. Effect of Cd and Ni on H2O2 Content, Lipid Peroxidation, and Protein Carbonylation of P. sativum Leaves
2.2. Effect of Cd and Ni on Reduced (GSH) and Oxidized Glutathione (GSSG) Contents of P. sativum Leaves
2.3. Effect of Cd and Ni on Antioxidant Enzymes Activity of P. sativum Leaves
2.4. Effect of Cd and Ni on Soluble Protein, Proline, and Phenolic Contents
2.5. Cd and Ni Contents in the Treated P. sativum
2.6. Alleviation Effect of ALA on H2O2, MDA, Protein Carbonylation, and Phenolic Contents in the P. sativum
3. Discussion
4. Materials and Methods
4.1. Treatment Experiment
4.2. Determination of H2O2 Content
4.3. Determination of Lipid Peroxidation
4.4. Determination of Protein and Protein Carbonylation
4.5. Determination of Glutathione
4.6. Antioxidant Enzymes Extraction and Assays
4.7. Proline Determination
4.8. Determination of Cd and Ni in P. sativum
4.9. Determination of Total Phenolics
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Weldeslassie, T.; Naz, H.; Singh, B.; Oves, M. Chemical contaminants for soil, air and aquatic ecosystem. In Modern Age Environmental Problems and their Remediation; Oves, M., Khan, M.Z., Ismail, I.M.I., Eds.; Springer: Basel, Switzerland, 2018; pp. 1–22. [Google Scholar]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sethy, S.K.; Ghosh, S. Effect of heavy metals on germination of seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272–275. [Google Scholar] [PubMed]
- Ahmad, M.S.A.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2012; pp. 125–167. [Google Scholar]
- De Maria, S.; Puschenreiter, M.; Rivelli, A. Cadmium accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. Plant Soil Environ. 2013, 59, 254–261. [Google Scholar] [CrossRef]
- Shanying, H.; Xiaoe, Y.; Zhenli, H.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar]
- Rahoui, S.; Chaoui, A.; El Ferjani, E. Membrane damage and solute leakage from germinating pea seed under cadmium stress. J. Hazard. Mater. 2010, 178, 1128–1131. [Google Scholar] [CrossRef]
- Smiri, M.; Chaoui, A.; Rouhier, N.; Gelhaye, E.; Jacquot, J.-P.; El Ferjani, E. Cadmium affects the glutathione/glutaredoxin system in germinating pea seeds. Biol. Trace Elem. Res. 2011, 142, 93–105. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, S.-H.; Lee, D.-G.; Lee, H.; Lee, S.W.; Bahk, J.D.; Lee, B.-H. Physiological and protein profiles alternation of germinating rice seedlings exposed to acute cadmium toxicity. C. R. Biol. 2007, 330, 735–746. [Google Scholar] [CrossRef]
- Celis-Hernandez, O.; Rosales-Hoz, L.; Cundy, A.B.; Carranza-Edwards, A.; Croudace, I.W.; Hernandez-Hernandez, H. Historical trace element accumulation in marine sediments from the Tamaulipas shelf, Gulf of Mexico: An assessment of natural vs. anthropogenic inputs. Sci. Total Environ. 2018, 622, 325–336. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma 2011, 248, 503–511. [Google Scholar] [CrossRef]
- El-Alfy, M.A.; El-Amier, Y.A.; AbdEl-Hamid, H.T. Soil quality and health risk assessment of heavy metals in agricultural areas irrigated with wastewater from Kitchener Drain, Nile Delta, Egypt. J. Sci. Agric. 2017, 1, 158–170. [Google Scholar] [CrossRef]
- Pervin, S.; Narayan, R.; Rahman, H. Effect of metal ions, chelating agent and SH-Reagents on Radish (Raphanus sativus L.) root β-amylase. J. Stress Physiol. Biochem. 2012, 8, 181–188. [Google Scholar]
- Elzebroek, A.T.G.; Wind, K. Guide to Cultivated Plants; CAB International: Oxford, UK, 2008. [Google Scholar]
- Khalifa, M.; Gad, A. Assessment of heavy metals contamination in agricultural soil of Southwestern Nile Delta, Egypt. Soil Sediment Contam. 2018, 27, 619–642. [Google Scholar] [CrossRef]
- Fernandes, J.; Henriques, F. Biochemical, physiological, and structural effects of excess copper in plants. Bot. Rev. 1991, 57, 246–273. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Zaid, A.; Arif, M.S.; Yasmeen, T.; Hussain, A.; Shahid, M.R.; Bukhari, S.A.H.; Hussain, S.; Abbasi, G.H. 5-Aminolevulinic acid-induced heavy metal stress tolerance and underlying mechanisms in plants. J. Plant Growth Regul. 2018, 37, 1423–1436. [Google Scholar] [CrossRef]
- Watanabe, K.; Tanaka, T.; Hotta, Y.; Kuramochi, H.; Takeuchi, Y. Improving salt tolerance of cotton seedlings with 5-aminolevulinic acid. Plant Growth Regul. 2000, 32, 97–101. [Google Scholar] [CrossRef]
- Xu, F.; Wang, W.; Yu, D. Effect of 5-aminolevulinic acid on yield and quality of lettuce in sunlit greenhouse. Afr. J. Biotechnol. 2012, 11, 11591–11594. [Google Scholar]
- Balestrasse, K.B.; Tomaro, M.L.; Batlle, A.; Noriega, G.O. The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 2010, 71, 2038–2045. [Google Scholar] [CrossRef]
- Cui, T.; Fang, L.; Wang, M.; Jiang, M.; Shen, G.J.J.o.C. Intercropping of gramineous pasture ryegrass (Lolium perenne L.) and leguminous forage alfalfa (Medicago sativa L.) increases the resistance of plants to heavy metals. J. Chem. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Lamhamdi, M.; Bakrim, A.; Aarab, A.; Lafont, R.; Sayah, F. Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. C. R. Biol. 2011, 334, 118–126. [Google Scholar] [CrossRef]
- Chmielowska-Bak, J.; Arasimowicz-Jelonek, M.; Izbianska, K.; Frontasyeva, M.; Zinicovscaia, I.; Guiance-Varela, C.; Deckert, J. NADPH oxidase is involved in regulation of gene expression and ROS overproduction in soybean (Glycine max L.) seedlings exposed to cadmium. ACTA Soc. Bot. Pol. 2017, 86, 1–17. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Pospíšil, P.; Yamamoto, Y. Damage to photosystem II by lipid peroxidation products. Biochim. Biophys. Acta -Gen. Subj. 2017, 1861, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Petukhov, A.S.; Khritokhin, N.A.; Petukhova, G.A. Lipid peroxidation in plants cells under conditions of the urban environment. RUDN J. Ecol. Life Safety 2018, 26, 82–90. [Google Scholar] [CrossRef]
- Malook, I.; Rehman, S.U.; Khan, M.D.; El-Hendawy, S.E.; Al-Suhaibani, N.A.; Aslam, M.M.; Jamil, M. Heavy metals induced lipid peroxidation in spinach mediated with microbes. Pak. J. Bot. 2017, 49, 2301–2308. [Google Scholar]
- Jimenez, A.; Hernandez, J.A.; del Río, L.A.; Sevilla, F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997, 114, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Khan, N.A. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Singapore, 2017. [Google Scholar]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Mendoza-Cozatl, D.; Loza-Tavera, H.; Hernández-Navarro, A.; Moreno-Sánchez, R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005, 29, 653–671. [Google Scholar] [CrossRef]
- Metwally, A.; Safronova, V.I.; Belimov, A.A.; Dietz, K.-J. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J. Exp. Bot. 2004, 56, 167–178. [Google Scholar] [CrossRef]
- Gautam, A.; Dubey, R.S. Metal toxicity in plants: Induction of oxidative stress, antioxidative defense system, metabolic alterations and phytoremediation. In Molecular Physiology of Abiotic Stresses in Plant Productivity; Hemantaranjan, A., Ed.; Scientific Publisher: Jodhpur, India, 2018; pp. 256–290. [Google Scholar]
- Alscher, R.G.; Erturk, N.; Heath, L. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Schatzman, S.S.; Culotta, V.C. Chemical warfare at the microorganismal level: A closer look at the superoxide dismutase enzymes of pathogens. ACS Infect. Dis. 2018, 4, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Cho, U.-H.; Park, J.-O. Mercury-induced oxidative stress in tomato seedlings. Plant Sci. 2000, 156, 1–9. [Google Scholar] [CrossRef]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Balabanova, D.; Remans, T.; Vassilev, A.; Cuypers, A.; Vangronsveld, J. Possible involvement of glutathione S-transferases in imazamox detoxification in an imidazolinone-resistant sunflower hybrid. J. Plant Physiol. 2018, 221, 62–65. [Google Scholar] [CrossRef]
- Prasad, K.; Saradhi, P.P.; Sharmila, P. Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ. Exp. Bot. 1999, 42, 1–10. [Google Scholar] [CrossRef]
- Prasad, M. Cadmium toxicity and tolerance in vascular plants. Environ. Exp. Bot. 1995, 35, 525–545. [Google Scholar] [CrossRef]
- El-Khallal, S.M.; Hathout, T.A.; Ahsour, A.; Kerrit, A. Brassinolide and salicylic acid induced antioxidant enzymes, hormonal balance and protein profile of maize plants grown under salt stress. Res. J. Agric. Biol. Sci. 2009, 5, 391–402. [Google Scholar]
- Stadtman, E.R.; Oliver, C.N. Metal-catalyzed oxidation of proteins. J. Biol. Chem. 1991, 266, 2005–2008. [Google Scholar]
- Singh, G.; Agnihotri, R.K.; Reshma, R.S.; Ahmad, M. Effect of lead and nickel toxicity on chlorophyll and proline content of Urd (Vigna mungo L.) seedlings. Int. J. Plant Physiol. Biochem. 2012, 4, 136–141. [Google Scholar]
- Tripathi, B.N.; Gaur, J.P. Relationship between copper-and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 2004, 219, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Zengin, F.K.; Munzuroglu, O. Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta. Biol. Cracov. Bot. 2005, 47, 157–164. [Google Scholar]
- Kiyosue, T.; Yoshiba, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 1996, 8, 1323–1335. [Google Scholar]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF, CF/5 INF/1; FAO/WHO: Hague, The Netherlands, 2011. [Google Scholar]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediat. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; Elshamy, A.; Al-Rowaily, S.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef] [Green Version]
- Maodzeka, A.; Wang, Q.; Chen, X.; Hussain, N.; Wu, D.; Jiang, L. Effects of 5-aminolevulinic acid on the bioactive compounds and seedling growth of oilseed rape (Brassica napus L.). J. Plant Biol. 2019, 62, 181–194. [Google Scholar] [CrossRef]
- Hoagland, D.; Arnon, D. The Water Culture Method for Growing Plant without Soil; California Agricultural Experiment Station: Davis, CA, USA, 1950. [Google Scholar]
- Zhou, B.; Wang, J.; Guo, Z.; Tan, H.; Zhu, X. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul. 2006, 49, 113–118. [Google Scholar] [CrossRef]
- Mandal, S.; Mitra, A.; Mallick, N. Biochemical characterization of oxidative burst during interaction between Solanum lycopersicum and Fusarium oxysporum f. sp. lycopersici. Physiol. Mol. Plant Pathol. 2008, 72, 56–61. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [PubMed]
- Anderson, M.P.; Gronwald, J.W. Atrazine resistance in a velvetleaf (Abutilon theophrasti) biotype due to enhanced glutathione S-transferase activity. Plant Physiol. 1991, 96, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miramar, M.D.; Costantini, P.; Ravagnan, L.; Saraiva, L.M.; Haouzi, D.; Brothers, G.; Penninger, J.M.; Peleato, M.L.; Kroemer, G.; Susin, S.A. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J. Biol. Chem. 2001, 276, 16391–16398. [Google Scholar] [CrossRef] [Green Version]
- Tórsdóttir, G.; Kristinsson, J.; Gudmundsson, G.; Snaedal, J.; Jóhannesson, T. Copper, ceruloplasmin and superoxide dismutase (SOD) in amyotrophic lateral sclerosis. Pharmacol. Toxicol. 2000, 87, 126–130. [Google Scholar] [CrossRef]
- Yan, J.; Meng, X.; Wancket, L.M.; Lintner, K.; Nelin, L.D.; Chen, B.; Francis, K.P.; Smith, C.V.; Rogers, L.K.; Liu, Y. Glutathione reductase facilitates host defense by sustaining phagocytic oxidative burst and promoting the development of neutrophil extracellular traps. J. Immunol. 2012, 188, 2316–2327. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK; London, UK, 1974. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods; NewAge International Limited: New Delhi, India, 2008. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |







| Parameters | Effect | SS | df | MS | F | P |
|---|---|---|---|---|---|---|
| MDA | Metal type | 340.06 | 1 | 340.06 | 156.42 | <0.0001 |
| Time of exposure | 6666.51 | 6 | 1111.08 | 511.07 | <0.0001 | |
| M × Time | 274.10 | 6 | 45.68 | 21.01 | <0.0001 | |
| Carbonyl | Metal type | 101.62 | 1 | 101.62 | 103.02 | <0.0001 |
| Time of exposure | 2632.91 | 6 | 438.82 | 444.87 | <0.0001 | |
| M × Time | 39.89 | 6 | 6.65 | 6.74 | <0.0002 | |
| H2O2 | Metal type | 438.76 | 1 | 438.76 | 161.61 | 0.0002 |
| Time of exposure | 10215.27 | 6 | 1702.54 | 627.11 | <0.0001 | |
| M × Time | 190.27 | 6 | 31.71 | 11.68 | <0.0001 | |
| GSH | Metal type | 1.58 | 1 | 1.58 | 6.45 | 0.0169 |
| Time of exposure | 478.20 | 6 | 79.70 | 325.03 | <0.0001 | |
| M × Time | 116.21 | 6 | 19.37 | 78.99 | <0.0001 | |
| GSSH | Metal type | 41.88 | 1 | 41.88 | 466.07 | <0.0001 |
| Time of exposure | 186.21 | 6 | 31.03 | 345.38 | <0.0001 | |
| M × Time | 19.28 | 6 | 3.21 | 35.75 | <0.0001 | |
| NADH-oxidase | Metal type | 48.75 | 1 | 48.75 | 104.19 | <0.0001 |
| Time of exposure | 1128.46 | 6 | 188.08 | 401.96 | <0.0001 | |
| M × Time | 13.33 | 6 | 2.22 | 4.75 | 0.0019 | |
| SOD | Metal type | 345.26 | 1 | 345.26 | 396.43 | <0.0001 |
| Time of exposure | 2549.24 | 6 | 424.87 | 487.84 | <0.0001 | |
| M × Time | 206.04 | 6 | 34.34 | 39.43 | <0.0001 | |
| CAT | Metal type | 157.72 | 1 | 157.72 | 126.22 | <0.0001 |
| Time of exposure | 3841.52 | 6 | 640.25 | 512.38 | <0.0001 | |
| M × Time | 75.81 | 6 | 12.63 | 10.11 | <0.0001 | |
| APX | Metal type | 56.9 | 1 | 56.93 | 217.48 | <0.0001 |
| Time of exposure | 794.44 | 6 | 132.41 | 505.79 | <0.0001 | |
| M × Time | 17.67 | 6 | 2.95 | 11.25 | <0.0001 | |
| GR | Metal type | 80.32 | 1 | 80.32 | 119.58 | <0.0001 |
| Time of exposure | 1142.34 | 6 | 190.39 | 283.45 | <0.0001 | |
| M × Time | 25.71 | 6 | 4.28 | 6.38 | 0.0003 | |
| Protein | Metal type | 57.77 | 1 | 57.77 | 82.80 | <0.0001 |
| Time of exposure | 1222.38 | 6 | 203.73 | 291.97 | <0.0001 | |
| M × Time | 13.78 | 6 | 2.30 | 3.29 | 0.0140 | |
| Proline | Metal type | 329.28 | 1 | 329.28 | 105.52 | <0.0001 |
| Time of exposure | 1553.87 | 6 | 258.98 | 82.99 | <0.0001 | |
| M × Time | 181.26 | 6 | 30.21 | 9.68 | <0.0001 | |
| Phenolics | Metal type | 236.01 | 1 | 236.01 | 287.96 | <0.0001 |
| Time of exposure | 1669.63 | 6 | 278.27 | 339.52 | <0.0001 | |
| M × Time | 54.18 | 6 | 9.03 | 11.02 | <0.0001 |
| Time (h) | Cd (mg Kg−1) | Ni (mg Kg−1) |
|---|---|---|
| 0 | ND | 0.050 ± 0.07 F |
| 12 | 0.013 ± 0.002 E | 0.110 ± 0.017 F |
| 24 | 0.020 ± 0.002 D | 0.306 ± 0.018 E |
| 36 | 0.032 ± 0.005 C | 0.630 ± 0.025 D |
| 48 | 0.047 ± 0.007 B | 1.887 ± 0.129 C |
| 60 | 0.062 ± 0.006 A | 2.902 ± 0.167 B |
| 72 | 0.065 ± 0.004 A | 3.355 ± 0.361 A |
| LSD0.05 | 0.005 | 0.168 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Amier, Y.; Elhindi, K.; El-Hendawy, S.; Al-Rashed, S.; Abd-ElGawad, A. Antioxidant System and Biomolecules Alteration in Pisum sativum under Heavy Metal Stress and Possible Alleviation by 5-Aminolevulinic Acid. Molecules 2019, 24, 4194. https://doi.org/10.3390/molecules24224194
El-Amier Y, Elhindi K, El-Hendawy S, Al-Rashed S, Abd-ElGawad A. Antioxidant System and Biomolecules Alteration in Pisum sativum under Heavy Metal Stress and Possible Alleviation by 5-Aminolevulinic Acid. Molecules. 2019; 24(22):4194. https://doi.org/10.3390/molecules24224194
Chicago/Turabian StyleEl-Amier, Yasser, Khalid Elhindi, Salah El-Hendawy, Sarah Al-Rashed, and Ahmed Abd-ElGawad. 2019. "Antioxidant System and Biomolecules Alteration in Pisum sativum under Heavy Metal Stress and Possible Alleviation by 5-Aminolevulinic Acid" Molecules 24, no. 22: 4194. https://doi.org/10.3390/molecules24224194