Constituents and Anti-Multidrug Resistance Activity of Taiwanofungus camphoratus on Human Cervical Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
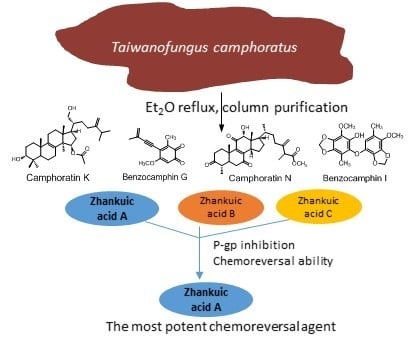
2.1. Purification and Identification of Chemical Constituents
2.2. Structural Elucidations of Camphoratins K (1) and N (2) and Benzocamphorins G (3) and I (4)
2.3. P-gp Inhibitory Effects of the Extract of T. camphoratus
2.4. Zhankuic Acids A–C Inhibited P-gp Efflux Function
2.5. The MDR Reversal Effects of ZAs A, B, C
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Extraction and Isolation
3.3.1. Camphoratin K (1)
3.3.2. Camphoratin N (2)
3.3.3. Benzocamphorin G (3)
3.3.4. Benzocamphorin I (4)
3.4. Culture of Cell Lines
3.5. Calcein AM Uptake Assay
3.6. SRB Cytotoxicity Assay and Reversal Fold Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Institute, N.C. Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 16 September 2019).
- Avril, T.; Vauleon, E.; Chevet, E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 2017, 6, e373. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016, 380, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimmakayala, R.K.; Batra, S.K.; Ponnusamy, M.P. Unraveling the Journey of Cancer Stem Cells from Origin to Metastasis. Biochim. Biophys. Acta Rev. Cancer 2018. [Google Scholar] [CrossRef]
- Sodani, K.; Patel, A.; Kathawala, R.J.; Chen, Z.S. Multidrug resistance associated proteins in multidrug resistance. Chin. J. Cancer 2012, 31, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Update 2015, 18, 1–17. [Google Scholar] [CrossRef]
- Palmeira, A.; Sousa, E.; Vasconcelos, M.H.; Pinto, M.M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef]
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Ther. 2013, 139, 124–156. [Google Scholar] [CrossRef]
- Lin, Y.H.; Kuo, J.T.; Chen, Y.Y.; Kumar, K.J.S.; Lo, C.P.; Lin, C.C.; Wang, S.Y. Immunomodulatory Effects of the Stout Camphor Medicinal Mushroom, Taiwanofungus camphoratus (Agaricomycetes)-Based Health Food Product in Mice. Int. J. Med. Mushrooms 2018, 20, 849–858. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lo, C.P.; Lin, C.C.; Hsieh, Y.H. Effects of Taiwanofungus camphoratus on non-specific and specific immune activities in mice. Mycology 2018, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, J.; Zhang, H.; Yang, Y.; Liu, Y.; Sun, W.; Wang, W.; Jia, W. Compound of Stout Camphor Medicinal Mushroom, Taiwanofungus camphoratus (Agaricomycetes), Induces Protective Autophagy in SPCA-1 Cells through AMPK Inhibition-Independent Blockade of the Akt/mTOR Pathway. Int. J. Med. Mushrooms 2018, 20, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.C.; Lai, G.M.; Chow, J.M.; Lee, H.L.; Yeh, C.F.; Li, C.H.; Yan, J.L.; Chuang, S.E.; Whang-Peng, J.; Bai, K.J.; et al. Active fraction (HS7) from Taiwanofungus camphoratus inhibits AKT-mTOR, ERK and STAT3 pathways and induces CDK inhibitors in CL1-0 human lung cancer cells. Chin. Med. 2017, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Wang, L.Y.; Yang, H.R.; Zhang, Y.P.; Zhang, H.N.; Fan, H.; Zhao, X.L.; Zhang, J.S.; Jia, W. Antitumor Effect of By-1 from Spent Broth from Submerged Cultures of Stout Camphor Medicinal Mushroom, Taiwanofungus camphoratus (Higher Basidiomycetes), on A549 Adenocarcinoma Cells. Int. J. Med. Mushrooms 2017, 19, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.J.; Shi, Y.C.; Tai, C.J.; Kuo, L.J.; Chen, R.J.; Chang, Y.J.; Tzao, C.; Wu, C.H.; Chang, C.C.; Chiou, H.Y.; et al. Taiwanofungus camphoratus Combined With Amphotericin B for Metastatic Cancer Patients Unresponsive to or Unwilling to Undergo Chemotherapy: A Pilot Study. Altern. Ther. Health Med. 2018. Available online: https://europepmc.org/abstract/med/29477137 (accessed on 15 October 2019).
- Wang, C.; Zhang, W.; Wong, J.H.; Ng, T.; Ye, X. Diversity of potentially exploitable pharmacological activities of the highly prized edible medicinal fungus Antrodia camphorata. Appl. Microbiol. Biotechnol. 2019, 103, 7843–7867. [Google Scholar] [CrossRef]
- Wu, S.J.; Leu, Y.L.; Chen, C.H.; Chao, C.H.; Shen, D.Y.; Chan, H.H.; Lee, E.J.; Wu, T.S.; Wang, Y.H.; Shen, Y.C.; et al. Camphoratins A–J, potent cytotoxic and anti-inflammatory triterpenoids from the fruiting body of Taiwanofungus camphoratus. J. Nat. Prod. 2010, 73, 1756–1762. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, X.; Qiao, X.; Ji, S.; Liu, K.; Yeh, C.T.; Tzeng, Y.M.; Guo, D.; Ye, M. Antcamphins A-L, ergostanoids from Antrodia camphorata. J. Nat. Prod. 2014, 77, 118–124. [Google Scholar] [CrossRef]
- Shi, L.S.; Chao, C.H.; Shen, D.Y.; Chan, H.H.; Chen, C.H.; Liao, Y.R.; Wu, S.J.; Leu, Y.L.; Shen, Y.C.; Kuo, Y.H.; et al. Biologically active constituents from the fruiting body of Taiwanofungus camphoratus. Bioorg. Med. Chem. 2011, 19, 677–683. [Google Scholar] [CrossRef]
- Wu, M.D.; Cheng, M.J.; Wang, W.Y.; Huang, H.C.; Yuan, G.F.; Chen, J.J.; Chen, I.S.; Wang, B.C. Antioxidant activities of extracts and metabolites isolated from the fungus Antrodia cinnamomea. Nat. Prod. Res. 2011, 25, 1488–1496. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiu, H.L.; Chao, C.Y.; Lin, W.H.; Chao, L.K.; Huang, G.J.; Kuo, Y.H. New Anti-Inflammatory Aromatic Components from Antrodia camphorata. Int. J. Mol. Sci. 2013, 14, 4629–4639. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lin, W.J.; Liao, C.H.; Shieh, P.C. Anti-inflammatory benzenoids from Antrodia camphorata. J. Nat. Prod. 2007, 70, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Shiao, Y.J.; Lin, R.D.; Shao, Y.Y.; Lai, M.N.; Lin, C.C.; Ng, L.T.; Kuo, Y.H. Neuroprotective diterpenes from the fruiting body of Antrodia camphorata. J. Nat. Prod. 2006, 69, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, C.K.; Tsou, W.L.; Liu, S.Y.; Kuo, M.T.; Wen, W.C. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata. Planta Med. 2007, 73, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.D.; Cheng, M.J.; Wang, B.C.; Yech, Y.J.; Lai, J.T.; Kuo, Y.H.; Yuan, G.F.; Chen, I.S. Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages. J. Nat. Prod. 2008, 71, 1258–1261. [Google Scholar] [CrossRef]
- Teng, Y.-N.; Chang, C.-S.; Lee, T.-E.; Hung, C.-C. Cordycepin re-sensitizes multidrug resistance cancer cells to chemotherapeutic agents through modulating P-glycoprotein expression and ATPase function. J. Funct. Foods 2016, 26, 681–690. [Google Scholar] [CrossRef]
- Teng, Y.N.; Hsieh, Y.W.; Hung, C.C.; Lin, H.Y. Demethoxycurcumin modulates human P-glycoprotein function via uncompetitive inhibition of ATPase hydrolysis activity. J. Agric. Food Chem. 2015, 63, 847–855. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the isolated compounds are available from the authors. |






| HeLa | KBvin | |||
|---|---|---|---|---|
| IC50 ± SD (nM) | RF 1 | IC50 ± SD (nM) | RF 1 | |
| Doxorubicin | 104.5 ± 6.36 | 1 | 3750 ± 70.7 | 1 |
| +Verapamil (2.5 μM) | 83.61 ± 3.12 * | 1.2 | 705.21 ± 19.13 * | 5.3 |
| +ZA-A (10 μM) | 76.000 ± 1.41 * | 1.4 | 420 ± 56.6 * | 8.9 |
| +ZA-A (20 μM) | 51.500 ± 2.12 * | 2 | 78.5 ± 3.53 * | 47.8 |
| +ZA-B (10 μM) | 103.000 ± 1.43 | 1 | 2050 ± 72.5 | 1.8 |
| +ZA-B (20 μM) | 66.500 ± 4.94 * | 1.6 | 1200 ± 23.5 * | 3.1 |
| +ZA-C (10 μM) | 101.500 ± 2.12 | 1 | 2100 ± 25.3 | 1.8 |
| +ZA-C (20 μM) | 83.000 ± 1.42 * | 1.3 | 1800 ± 45.7 * | 2.1 |
| Paclitaxel | 4.65 ± 0.21 | 1 | 1824 ± 125.87 | 1 |
| +Verapamil (2.5 μM) | 0.95 ± 0.03 * | 4.9 | 75.81 ± 4.95 * | 24.1 |
| +ZA-A (10 μM) | 1.650 ± 0.07 * | 2.8 | 143.5 ± 4.94 * | 12.7 |
| +ZA-A (20 μM) | 0.450 ± 0.08 * | 10.3 | 48.5 ± 2.12 * | 37.6 |
| +ZA-B (10 μM) | 1.900 ± 0.14 * | 2.4 | 228.5 ± 2.23 * | 8 |
| +ZA-B (20 μM) | 0.750 ± 0.07 * | 6.2 | 141.5 ± 4.78 * | 12.9 |
| +ZA-C (10 μM) | 4.000 ± 0.28 | 1.2 | 253.6 ± 5.16 * | 7.2 |
| +ZA-C (20 μM) | 3.700 ± 0.56 | 1.3 | 221.8 ± 2.54 * | 8.2 |
| Vincristine | 41.5 ± 0.74 | 1 | 14540 ± 719.13 | 1 |
| +Verapamil (2.5 μM) | 37.9 ± 0.64 | 1.1 | 370.81 ± 8.34 * | 39.2 |
| +ZA-A (10 μM) | 6.450 ± 1.06 * | 6.4 | 2187 ± 30.7 * | 6.6 |
| +ZA-A (20 μM) | 3.450 ± 0.77 * | 12 | 321.5 ± 3.53 * | 45.2 |
| +ZA-B (10 μM) | 8.350 ± 1.17 * | 5 | 2252 ± 11.31 * | 6.5 |
| +ZA-B (20 μM) | 5.700 ± 0.98 * | 7.3 | 1355.5 ± 30.41 * | 10.7 |
| +ZA-C (10 μM) | 31.500 ± 2.47 | 1.3 | 2484 ± 55.15 * | 5.9 |
| +ZA-C (20 μM) | 16.500 ± 0.71 * | 2.5 | 971.5 ± 37.8 * | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-Y.; Hung, C.-C.; Liang, J.-W.; Chen, C.-F.; Chen, H.-Y.; Shieh, P.-C.; Kuo, P.-C.; Wu, T.-S. Constituents and Anti-Multidrug Resistance Activity of Taiwanofungus camphoratus on Human Cervical Cancer Cells. Molecules 2019, 24, 3730. https://doi.org/10.3390/molecules24203730
Hung H-Y, Hung C-C, Liang J-W, Chen C-F, Chen H-Y, Shieh P-C, Kuo P-C, Wu T-S. Constituents and Anti-Multidrug Resistance Activity of Taiwanofungus camphoratus on Human Cervical Cancer Cells. Molecules. 2019; 24(20):3730. https://doi.org/10.3390/molecules24203730
Chicago/Turabian StyleHung, Hsin-Yi, Chin-Chuan Hung, Jun-Weil Liang, Chin-Fu Chen, Hung-Yi Chen, Po-Chuen Shieh, Ping-Chung Kuo, and Tian-Shung Wu. 2019. "Constituents and Anti-Multidrug Resistance Activity of Taiwanofungus camphoratus on Human Cervical Cancer Cells" Molecules 24, no. 20: 3730. https://doi.org/10.3390/molecules24203730