It’s Only a Part of the Story: Analytical Investigation of the Inks and Dyes Used in the Privilegium Maius
Abstract
:1. Introduction
The History of the Privilegium Maius
2. Results
2.1. Analysis of the Inks
2.2. Analysis of the Coloured Threads—Non-Invasive Measurements
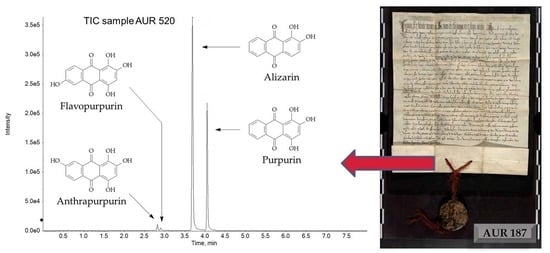
2.2.1. Purple Threads
- One thread (AUR 1845) could be dyed with orchil, a lichen dye, as suggested by the two weak absorption bands between 540 and 590 nm [5];
- The threads of AUR 187, AUR 520, AUR 708, AUR 1359 VIII 10, AUR 1360 VII 11 and possibly AUR 1844, could be dyed with anthraquinones from plants, according to the presence of two absorption bands between 500 and 540 nm [6];
- One thread (AUR 1792) could be dyed with a modern colourant, considering the unusual spectral features and in particular the fluorescence emission at high wavelength. The term modern is to be referred to a re-dyeing process or to the substitution of the original thread with a modern one; indeed, we had no clue for the identification of the original purple colourant of this thread.
2.2.2. Green Threads
2.2.3. Preliminary Results
2.3. Analysis of the Coloured Threads—Micro-Invasive Approach
2.3.1. Purple Threads
2.3.2. Yellow Threads
2.3.3. Green Threads
3. Discussion
3.1. Analysis of the Inks
3.2. Analysis of the Dyes Used for the Threads
4. Materials and Methods
4.1. Reagents
4.2. UV–Visible Diffuse Reflectance Spectrophotometry with Fibre Optic (FORS) Analysis
4.3. Molecular Spectrofluorimetry with Fibre Optic (FOMF) Analysis
4.4. Raman Analysis
4.5. HPLC-DAD-MS Analysis
4.6. UHPLC-MS/MS Analysis
4.7. Extraction Procedures
- for purple threads, a mixture formic acid/methanol/water (FMW) 2:1:1 was used, 10 min at 90 °C;
- for yellow threads and the yellow component of green threads, the same FMW mixture was used, 5 min at 80 °C;
- for the blue component of green threads: DMSO, 10 min at 90 °C.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Just, T. Geschichte wird gemacht. Von Herzog Rudolf IV. zu Heinz Grill: Das Privilegium maius im Archiv. In Privilegium maius. Autopsie, Kontext und Karriere der Fälschungen Rudolfs IV. von Österreich; Just, T., Kininger, K., Sommerlechner, A., Weigl, H., Eds.; Böhlau Verlag: Wien, Austria, 2018; pp. 25–40. [Google Scholar]
- Aceto, M.; Agostino, A.; Boccaleri, E.; Garlanda, A.C. The Vercelli Gospels laid open: an investigation into the inks used to write the oldest Gospels in Latin. X-Ray Spectrom. 2008, 37, 286–292. [Google Scholar] [CrossRef]
- Moorhead, G.; Mazzarino, S.; Marzo, F.; Knight, B. A physical perspective of Codex Sinaiticus: an overview from British Library. In Codex Sinaiticus: New Perspectives on the Ancient Biblical Manuscript; McKendrick, S., Parker, D., Myshrall, A., O’Hogan, C., Eds.; The British Library: London, UK, 2015; pp. 221–238. [Google Scholar]
- Aceto, M.; Calà, E. Analytical evidences of the use of iron-gall ink as a pigment on miniature paintings. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Arrais, A.; Marsano, F.; Agostino, A.; Fenoglio, G.; Idone, A.; Gulmini, M. A diagnostic study on folium and orchil dyes with non-invasive and micro-destructive methods. Spectrochim. Acta A 2015, 142, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Gulmini, M.; Idone, A.; Davit, P.; Moi, M.; Carrillo, M.; Ricci, C.; Bello, F.D.; Borla, M.; Oliva, C.; Greco, C.; et al. The “Coptic” textiles of the “Museo Egizio” in Torino (Italy): A focus on dyes through a multi-technique approach. Archaeol. Anthropol. Sci. 2017, 9, 485–497. [Google Scholar] [CrossRef]
- Hofenk de Graaff, J.H. The Colourful Past; Origins, Chemistry and Identification of Natural Dyestuffs; Archetype Publications: London, UK, 2004. [Google Scholar]
- Caley, E.R. The Stockholm Papyrus. An English translation with brief notes. J. Chem. Educ. 1927, 4, 979. [Google Scholar] [CrossRef]
- Aceto, M.; Agostino, A.; Fenoglio, G.; Idone, A.; Crivello, F.; Griesser, M.; Kirchweger, F.; Uhlir, K.; Puyo, P.R. Analytical investigations on the Coronation Gospels manuscript. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Leona, M.; Vo-Dinh, K.-C.; Yan, F.; Wabuyele, M.B.; Vo-Dinh, T. Application of surface-enhanced Raman scattering (SERS) for the identification of anthraquinone dyes used in works of art. J. Raman Spectrosc. 2006, 37, 520–527. [Google Scholar] [CrossRef]
- Bruni, S.; Guglielmi, V.; Pozzi, F. Historical organic dyes: A surface-enhanced Raman scattering (SERS) spectral database on Ag Lee-Meisel colloids aggregated by NaClO4. J. Raman Spectrosc. 2011, 42, 1267–1281. [Google Scholar] [CrossRef]
- Graebe, C.; Liebermann, C. Ueber künstliche Bildung von Alizarin. Berichte der Dtsch. Chem. Gesellschaft 1869, 2, 14. [Google Scholar] [CrossRef]
- Wouters, J.; Vanden Berghe, I.; Richard, G.; Breniaux, R.; Cardon, D. Dye analysis of selected textiles from three Roman sites in the Eastern Desert of Egypt: A hypothesis on the dyeing technology in Roman and Coptic Egypt. Dye. Hist. Archaeol. 2008, 21, 1–16. [Google Scholar]
- Mouri, C.; Laursen, R. Identification of anthraquinone markers for distinguishing Rubia species in madder-dyed textiles by HPLC. Microchim. Acta 2012, 179, 105–113. [Google Scholar] [CrossRef]
- Boldizsár, I.; Szűcs, Z.; Füzfai, Z.; Molnár-Perl, I. Identification and quantification of the constituents of madder root by gas chromatography and high-performance liquid chromatography. J. Chromatogr. A 2006, 1133, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.L.; Rayner, C.M.; Blackburn, R.S. Isolation and extraction of lucidin primeveroside from Rubia tinctorum L. and crystal structure elucidation. Phytochemistry 2013, 95, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, L.; Rayner, C.M.; Blackburn, R.S. Isolation and extraction of ruberythric acid from Rubia tinctorum L. and crystal structure elucidation. Phytochemistry 2015, 117, 168–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhou, Y.; Zhao, F.; Peng, Z.; Wang, S. Identification of early synthetic dyes in historical Chinese textiles of the late nineteenth century by high-performance liquid chromatography coupled with diode array detection and mass spectrometry. Color. Technol. 2016, 132, 177–185. [Google Scholar] [CrossRef]
- Wertz, J.; Quye, A.; France, D.; Tang, P.L.; Richmond, L. Authenticating Turkey red textiles through material investigations by FTIR and UHPLC. In Proceedings of the Proceedings of the ICOM-CC 18th Triennial Meeting, Copenhagen, Denmark, September 2017; pp. 1–8. [Google Scholar]
- Singh, R.; Geetanjali; Chauhan, S.M.S. 9,10-Anthraquinones and Other Biologically Active Compounds from the GenusRubia. Chem. Biodivers. 2004, 1, 1241–1264. [Google Scholar] [CrossRef]
- Cardon, D. Natural Dyes: Sources, Tradition, Technology and Science; Archetype Books: London, UK, 2007. [Google Scholar]
- Peggie, D.A.; Hulme, A.N.; McNab, H.; Quye, A. Towards the identification of characteristic minor components from textiles dyed with weld (Reseda luteola L.) and those dyed with Mexican cochineal (Dactylopius coccus Costa). Microchim. Acta 2008, 162, 371–380. [Google Scholar] [CrossRef]
- Witkowski, B.; Ganeczko, M.; Hryszko, H.; Stachurska, M.; Gierczak, T.; Biesaga, M. Identification of orcein and selected natural dyes in 14th and 15th century liturgical paraments with high-performance liquid chromatography coupled to the electrospray ionization tandem mass spectrometry (HPLC-ESI/MS/MS). Microchem. J. 2017, 133, 370–379. [Google Scholar] [CrossRef]
- Griesser, M.; Uhlir, K.; Pitthard, V.; Strolz, M.; Eder, M.; Uldrich, A.; Aumüller, M. Strahlendiagnostische und materialanalytische Untersuchungen zum Urkundenkomplex „Privilegium maius“. In Privilegium Maius. Autopsie, Kontext und Karriere der Fälschungen Rudolfs IV. von Österreich; Just, T., Kininger, K., Sommerlechner, A., Weigl, H., Eds.; Böhlau Verlag: Wien, Austria, 2018; pp. 41–56. [Google Scholar]
- Aceto, M.; Agostino, A.; Fenoglio, G.; Capra, V.; Demaria, E.; Cancian, P. Characterisation of the different hands in the composition of a 14th century breviary by means of portable XRF analysis and complementary techniques. X-Ray Spectrom. 2017, 46, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Idone, A.; Gulmini, M.; Henry, A.-I.; Casadio, F.; Chang, L.; Appolonia, L.; Van Duyne, R.P.; Shah, N.C. Silver colloidal pastes for dye analysis of reference and historical textile fibers using direct, extractionless, non-hydrolysis surface-enhanced Raman spectroscopy. Analyst 2013, 138, 5895–5903. [Google Scholar] [CrossRef]
- Brosseau, C.L.; Casadio, F.; Van Duyne, R.P. Revealing the invisible: Using surface-enhanced Raman spectroscopy to identify minute remnants of color in Winslow Homer’s colorless skies. J. Raman Spectrosc. 2011, 42, 1305–1310. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







| Charter | Item Analysed | Group | Ink Type |
|---|---|---|---|
| AUR 98 | ink | Privilegium maius | IGI 1, IGI 1/carbon |
| AUR 187 | ink, dyed threads | Privilegium maius | IGI 1 |
| AUR 520 | ink, dyed threads | Privilegium maius | IGI 1 |
| AUR 708 | ink, dyed threads | Privilegium maius | IGI1 |
| AUR 1845 | ink, dyed threads | Privilegium maius | IGI 1 |
| AUR 97 | ink | comparative data | IGI 1 |
| AUR 1359 VIII 10 | ink, dyed threads | comparative data | IGI 1 |
| AUR 1360 VII 11 | ink, dyed threads | comparative data | IGI 1, IGI 1/carbon |
| AUR 1792 | ink, dyed threads | comparative data | IGI 1 |
| AUR 1844 | dyed threads | comparative data | 2 |
| Charter | Thread Colour | Identification by FORS/FOMF |
|---|---|---|
| AUR 187 PM | purple | anthraquinone dye |
| AUR 520 PM | purple | anthraquinone dye |
| AUR 708 PM | purple | anthraquinone dye |
| AUR 1845 PM | green | indigo/yellow dye |
| purple | lichen dye | |
| yellow | n.i. 1 | |
| AUR 1359 VIII 10 cd | green | indigo/yellow dye |
| purple | anthraquinone dye | |
| AUR 1360 VII 11 cd | green | indigo/yellow dye |
| purple | anthraquinone dye | |
| AUR 1792 cd | purple | modern dye |
| yellow | modern dye | |
| AUR 1844 cd | purple | n.i. 1 |
| Compound | AUR 187 | AUR 520 | AUR 708 | AUR 1845 | AUR 1359 VIII 10 | AUR 1360 VII 11 |
|---|---|---|---|---|---|---|
| alizarin | 100.0 | 100.0 | 100.0 | 1 | 100.0 | 100.0 |
| munjistin | 37.4 | 45.5 | 75.62 | 1 | 11.8 | 8.6 |
| purpurin | 4,8 | 1.9 | 21.5 | 1 | 16.4 | 9.9 |
| rubiadin | 45.5 | 12.1 | 1.1 | 1 | 2.8 | 119.7 |
| Compound | Q1 | Q3 | Time (min) | DP 1 (V) | EP 2 (V) | CE 3 V) | CXP 4 (V) |
|---|---|---|---|---|---|---|---|
| Alizarin 1 | 241.1 | 139.1 | 3.7 | 59.2 | 3.1 | 55.4 | 2.3 |
| Alizarin 2 | 241.1 | 157.1 | 3.7 | 59.2 | 3.1 | 36.0 | 2.5 |
| Alizarin 3 | 241.1 | 128.2 | 3.7 | 59.2 | 3.1 | 62.1 | 2.2 |
| Alizarin 4 | 241.1 | 129.1 | 3.7 | 59.2 | 3.1 | 42.9 | 2.2 |
| Flavopurpurin + Anthrapurpurin 1 | 257.1 | 173.1 | 2.8 | 62.0 | 10.0 | 36.7 | 2.8 |
| Flavopurpurin + Anthrapurpurin 2 | 257.1 | 155.1 | 2.8 | 62.0 | 10.0 | 51.8 | 2.4 |
| Flavopurpurin + Anthrapurpurin 3 | 257.1 | 127.1 | 2.8 | 62.0 | 10.0 | 60.4 | 2.2 |
| Flavopurpurin + Anthrapurpurin 4 | 257.1 | 145.1 | 2.8 | 62.0 | 10.0 | 43.2 | 2.3 |
| Purpurin 1 | 257.1 | 77.1 | 4.0 | 56.9 | 3.9 | 79.0 | 1.9 |
| Purpurin 2 | 257.1 | 229.1 | 4.0 | 56.9 | 3.9 | 33.0 | 2.5 |
| Purpurin 3 | 257.1 | 187.1 | 4.0 | 56.9 | 3.9 | 33.0 | 2.5 |
| Purpurin 4 | 257.1 | 127.1 | 4.0 | 56.9 | 3.9 | 59.1 | 2.2 |
| Charter | Thread Colour | Final Identification | Possible Dyeing Period |
|---|---|---|---|
| AUR 187 | purple | (1) madder from Rubia tinctorum (2) synthetic alizarin | (1) medieval (2) modern (19th century) |
| AUR 520 | purple | (1) madder from Rubia tinctorum (2) synthetic alizarin | (1) medieval (2) modern (19th century) |
| AUR 708 | purple | (1) madder from Rubia tinctorum (2) synthetic alizarin | (1) medieval (2) modern (19th century) |
| AUR 1845 | green | woad/ weld from Reseda luteola | medieval |
| purple | orchil | medieval | |
| yellow | weld from Reseda luteola | medieval | |
| AUR 1359 VIII 10 | green | woad/weld from Reseda luteola | medieval |
| purple | (1) madder from Rubia tinctorum (2) synthetic alizarin | (1) medieval (2) modern (19th century) | |
| AUR 1360 VII 11 | green | woad/weld from Reseda luteola | medieval |
| purple | (1) madder from Rubia tinctorum (2) synthetic alizarin | (1) medieval (2) modern (19th century) | |
| AUR 1792 | purple | Permanent Bordeaux FGR | modern (20th century) |
| yellow | n.i. 1 | possibly modern | |
| AUR 1844 | purple | n.i. 1 | possibly modern |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calà, E.; Gosetti, F.; Gulmini, M.; Serafini, I.; Ciccola, A.; Curini, R.; Salis, A.; Damonte, G.; Kininger, K.; Just, T.; et al. It’s Only a Part of the Story: Analytical Investigation of the Inks and Dyes Used in the Privilegium Maius. Molecules 2019, 24, 2197. https://doi.org/10.3390/molecules24122197
Calà E, Gosetti F, Gulmini M, Serafini I, Ciccola A, Curini R, Salis A, Damonte G, Kininger K, Just T, et al. It’s Only a Part of the Story: Analytical Investigation of the Inks and Dyes Used in the Privilegium Maius. Molecules. 2019; 24(12):2197. https://doi.org/10.3390/molecules24122197
Chicago/Turabian StyleCalà, Elisa, Fabio Gosetti, Monica Gulmini, Ilaria Serafini, Alessandro Ciccola, Roberta Curini, Annalisa Salis, Gianluca Damonte, Kathrin Kininger, Thomas Just, and et al. 2019. "It’s Only a Part of the Story: Analytical Investigation of the Inks and Dyes Used in the Privilegium Maius" Molecules 24, no. 12: 2197. https://doi.org/10.3390/molecules24122197