Synthesis and Comparative Evaluation of Novel Cationic Amphiphile C12-Man-Q as an Efficient DNA Delivery Agent In Vitro
Abstract
:1. Introduction
2. Result and Discussion
2.1. Synthesis of Cationic 1,4-Dihydropyridine Derivatives D19 and C12-Man-Q
2.2. Choice of Plasmid DNA
2.3. Evaluation of Complexation Ability
2.4. Cytotoxicoty Evaluation of 1,4-DHP/DNA Complexes
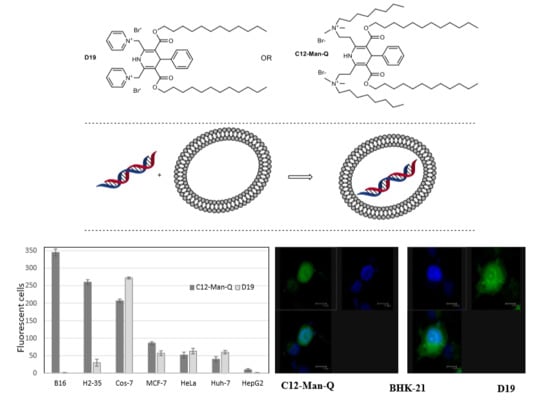
2.5. Evaluation of Transfection Activity
2.6. Cell Line-Dependent Differences in Transfection Efficiency of the New Amphiphilic 1,4-DHP Derivative C12-Man-Q
3. Materials and Methods
3.1. General
3.2. Synthesis of compounds 1, 2 and D19 (Scheme 1)
- Didodecyl 2,6-bis-(2-(N,N-dimethylamino)ethyl)-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate (3). A mixture of didodecyl 3,5-bis(dodecyloxycarbonyl)-2,6-dimethyl-4-phenyl-1,4-dihydropyridine (1, 1.22 g, 2.00 mmol), paraformaldehyde (0.40 g, 0.01 eq, 0.02 mol), dimethylamine hydrochloride (0.98 g, 12.0 mmol), a conc. HCl (0.150 mL) and ethanol (10 mL) was refluxed for 24 h. The solvent was removed by evaporation in vacuo, after which water (5 mL) was added. The pH of the resulting mixture was adjusted to approx. 8 with an aqueous solution of sodium carbonate and extracted with chloroform (6 × 30 mL). The organic layer was washed with brine and dried over MgSO4. After removal of the solvent, the oily residue was obtained and purified by flash chromatography (eluent: chloroform:acetone:methanol:Et3N = 9:4:2:0.02) yielding 0.75 g (68%) of light yellow foam. TLC: Rf: 0.2 (CHCl3/MeOH/Et3N = 1:1:0.01). 1H-NMR (CDCl3): δ 0.86–0.90 (m, 6H), 1.23–1.34 (m, 36H); 1.55–1.60 (m, 4H), 2.33 (s, 12H), 2.52–2.66 (m, 4H), 2.90–3.09 (m, 4H), 3.98–4.02 (m, 4H), 4.98 (s, 1H), 7.08–7.12 (m, 1H), 7.16–7.20 (m, 2H), 7.26–7.27 (m, 2H), 10.13 (s, 1H) ppm. 13C-NMR (CDCl3): δ 14.1, 18.4, 22.7, 26.1, 27.8, 28.7, 29.3, 29.4, 29.5, 29.6, 29.7, 31.9, 37.0, 39.4, 44.7, 63.8, 69.2, 102.8, 125.9, 127.7, 127.8, 148.2, 148.3, 167.7 ppm. MS (+ESI) m/z (relative intensity); 725 ([M + H]+, 60). Anal. calcd. for C45H77N3O4: C, 74.64; H, 10.72; N, 5.80; Found: C, 74.49; H, 10.55; N, 5.91.
- N,N′-{[3,5-Bis(dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl]diethylene}bis N,N-dimethyoctyl ammonium dibromide (C12-Man-Q). A mixture of compound 3 (290 mg, 0.4 mmol, 1 eq) and 1-bromooctane (232 mg, 1.2 mmol, 3 eq) in nitromethane (3 mL) and DMF (0.5 mL) was refluxed under argon for 48 h. The solvents were removed in vacuo and the residue was triturated with a small amount of dry acetone. After cooling the precipitate was filtered off and crystallised from dry acetone yielding compound C12-Man-Q as a light yellow powder (222 mg, 50%), mp 180–183 °C. 1H-NMR (CDCl3): δ 0.79–0.84 (m, 12H), 1.21–1.30 (m, 56H), 1.53 (br s, 4H), 1.60–1.87 (m, 8H), 3.22–3.38 (m, 2H)overlap, 3.36 (s, 12H)overlap, 3.48–3.56 (m, 2H), 3.65–3.73 (m, 2H), 3.85–3.97 (m, 4H), 4.24–4.35 (m, 2H), 4.74 (s, 1H), 7.04–7.08 (m, 1H), 7.12–7.15 (m, 2H), 7.20–7.26 (m, 2H)overlap with CDCl3, 10.02 (s, 1H) ppm. 13C-NMR (CDCl3): δ 14.0, 14.1, 22.5, 22.6, 29.3, 29.6, 29.7, 31.9, 39.2, 51.3, 51.8, 61.6, 64.1, 64.7, 105.4, 126.4, 127.9, 128.0, 143.6, 147.2, 166.9 ppm. MS (+ESI) m/z (relative intensity); 950 ([M-2Br + H]+ 10). Anal. calcd. for C61H111Br2N3O4: C, 65.98; H, 10.08; N, 3.78; Found: C, 65.71; H, 10.15; N, 3.72.
3.3. Calculation of Nitrogen to Phosphorus (N/P) Ratio
3.4. Preparation of Amphiphile/pDNA Complexes (Lipoplexes)
3.5. In Vitro Transfection Experiment
3.5.1. Cytotoxicity Assay
3.5.2. Assessment of Transfection’s Efficiency
3.5.3. Sample Preparation for Confocal Laser Scanning Microscopy
3.5.4. Sample Preparation for Transmission Electron Microscopy Studies
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Wirth, T.; Parker, N.; Ylä-Herttuala, S. History of gene therapy. Gene 2013, 525, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Foldvari, M.; Chen, D.W.; Nafissi, N.; Calderon, D.; Narsineni, L.; Rafiee, A. Non-viral gene therapy: Gains and challenges of non-invasive administration methods. J. Control. Release 2016, 240, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.M.; ElMallah, M.K.; Flotte, T.R. Gene Therapy 2017: Progress and Future Directions. Clin. Transl. Sci. 2017, 10, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, D.B.T.; Platt, R.J.; Zhang, F. Therapeutic genome editing: Prospects and challenges. Nat. Med. 2015, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of Recombinant DNA Technology to Improve Life. Int. J. Genom. 2016, 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.; Wagner, E. Therapeutic plasmid DNA versus siRNA delivery: Common and different tasks for synthetic carriers. J. Control. Release 2012, 161, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kievit, F.M.; Zhang, M. Nanoparticles for cancer gene therapy: Recent advances, challenges, and strategies. Pharmacol. Res. 2016, 114, 56–66. [Google Scholar] [CrossRef] [PubMed]
- El-Aneed, A. Current strategies in cancer gene therapy. Eur. J. Pharmacol. 2004, 498, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morille, M.; Passirani, C.; Vonarbourg, A.; Clavreul, A.; Benoit, J.-P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 2008, 29, 3477–3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Huang, L. Nonviral gene therapy: Promises and challenges. Gene Ther. 2000, 7, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.B.; Northeved, H.; Kumar Ek, P.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine 2015, 11, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anson, D.S. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet. Vaccines Ther. 2004, 2, 9–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.-L.; Ertl, H.C.J. Immune barriers to successful gene therapy. Trends Mol. Med. 2009, 15, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Keles, E.; Song, Y.; Du, D.; Dong, W.-J.; Lin, Y. Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 2016, 4, 1291–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, X.; Zhu, D.; Wang, Y.; Zhang, Z.; Zhou, X.; Qiu, N.; Chen, X.; Shen, Y. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. Adv. Drug Deliv. Rev. 2017, 115, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.P.; Arabinda, C. Cationic liposomes as non-viral carriers of gene medicines: Resolved issues, open questions, and future promises. Med. Res. Rev. 2007, 27, 696–722. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B. Enhancing nucleic acid delivery, insights from the cationic phospholipid carriers. Curr. Pharm. Biotechnol. 2014, 15, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, P.; Jubeli, E.; Raju, L.; Khalique, N.A.; Almeer, A.; Allam, H.; Manaa, M.A.; Larsen, H.; Nicholson, D.; Pungente, M.D.; et al. Aspects of nonviral gene therapy: Correlation of molecular parameters with lipoplex structure and transfection efficacy in pyridinium-based cationic lipids. Int. J. Pharm. 2014, 461, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Hiwale, A.A.; Voshavar, C.; Dharmalingam, P.; Dhayani, A.; Mukthavaram, R.; Nadella, R.; Sunnapu, O.; Gandhi, S.; Naidu, V.G.M.; Chaudhuri, A.; et al. Scaling the effect of hydrophobic chain length on gene transfer properties of di-alkyl, di-hydroxy ethylammonium chloride based cationic amphiphiles. RSC Adv. 2017, 7, 25398–25405. [Google Scholar] [CrossRef] [Green Version]
- Tirzite, D.; Koronova, J.; Plotniece, A. Influence of some quaternised 1,4-dihydropyridine derivatives on liposomes and erythrocyte membranes. Biochem. Mol. Biol. Int. 1998, 45, 849–856. [Google Scholar] [PubMed]
- Hyvonen, Z.; Plotniece, A.; Reine, I.; Chekavichus, B.; Duburs, G.; Urtti, A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim. Biophys. Acta Biomembr. 2000, 1509, 451–466. [Google Scholar] [CrossRef]
- Pajuste, K.; Hyvonen, Z.; Petrichenko, O.; Kaldre, D.; Rucins, M.; Cekavicus, B.; Ose, V.; Skrivele, B.; Gosteva, M.; Morin-Picardat, E.; et al. Gene delivery agents possessing antiradical activity: Self-assembling cationic amphiphilic 1,4-dihydropyridine derivatives. New J. Chem. 2013, 37, 3062–3075. [Google Scholar] [CrossRef]
- Petrichenko, O.; Rucins, M.; Vezane, A.; Timofejeva, I.; Sobolev, A.; Cekavicus, B.; Pajuste, K.; Plotniece, M.; Gosteva, M.; Kozlovska, T.; et al. Studies of the physicochemical and structural properties of self-assembling cationic pyridine derivatives as gene delivery agents. Chem. Phys. Lipids 2015, 191, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Pajuste, K.; Plotniece, A.; Kore, K.; Intenberga, L.; Cekavicus, B.; Kaldre, D.; Duburs, G.; Sobolev, A. Use of pyridinium ionic liquids as catalysts for the synthesis of 3,5-bis(dodecyloxycarbonyl)-1,4-dihydropyridine derivative. Cent. Eur. J. Chem. 2011, 9, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Aritomi, J.; Ueda, S.; Nishimura, H. Mannich Reaction of Dihydropyridine Derivatives. I. Reactions with Secondary Amines. Chem. Pharm. Bull. 1980, 28, 3163–3171. [Google Scholar] [CrossRef]
- Both compounds were analysed by HPLC on a Zorbax CN (5 µm,4.6 × 250 mm, Agilent, Santa Clara, CA, USA) column using a mobile phase of acetonitrile/0.05 M phosphate buffer, pH 2 (80:20 by volume) at a flow rate of 1.5 mL/min with detection at UV 210 nm. Under the conditions analysed, the retention times of D-19 and C12-Man-Q were 5.5 and 3.3 min, respectively.
- Hardee, C.L.; Arévalo-Soliz, L.M.; Hornstein, B.D.; Zechiedrich, L. Advances in non-viral DNA vectors for gene therapy. Genes 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, L.; Yuan, Z.; Węgrzyn, G.; Węgrzyn, A. Classification of plasmid vectors using replication origin, selection marker and promoter as criteria. Plasmid 2009, 61, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Román, R.; Miret, J.; Scalia, F.; Casablancas, A.; Lecina, M.; Cairó, J.J. Enhancing heterologous protein expression and secretion in HEK293 cells by means of combination of CMV promoter and IFNα2 signal peptide. J. Biotechnol. 2016, 239, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Prasher, D.C.; Eckenrode, V.K.; Ward, W.W.; Prendergast, F.G.; Cormier, M.J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 1992, 111, 229–233. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green fluorescent protein as a marker for gene expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Tsuji, F.I. Evidence for redox forms of the Aequorea green fluorescent protein. FEBS Lett. 1994, 351, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Van Gaal, E.V.B.; van Eijk, R.; Oosting, R.S.; Kok, R.J.; Hennink, W.E.; Crommelin, D.J.A.; Mastrobattista, E. How to screen non-viral gene delivery systems in vitro? J. Control. Release 2011, 154, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, E.V.B.; Oosting, R.S.; van Eijk, R.; Bakowska, M.; Feyen, D.; Kok, R.J.; Hennink, W.E.; Crommelin, D.J.A.; Mastrobattista, E. DNA Nuclear Targeting Sequences for Non-Viral Gene Delivery. Pharm. Res. 2011, 28, 1707–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofland, H.E.; Shephard, L.; Sullivan, S.M. Formation of stable cationic lipid/DNA complexes for gene transfer. Proc. Natl. Acad. Sci. USA 1996, 93, 7305–7309. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.R.; Chan, C.K. Differential intracellular distribution of DNA complexed with polyethylenimine (PEI) and PEI-polyarginine PTD influences exogenous gene expression within live COS-7 cells. Genet. Vaccines Ther. 2007, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De los Milagros Bassani Molinas, M.; Beer, C.; Hesse, F.; Wirth, M.; Wagner, R. Optimizing the transient transfection process of HEK-293 suspension cells for protein production by nucleotide ratio monitoring. Cytotechnology 2014, 66, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Even-Chen, S.; Cohen, R.; Barenholz, Y. Factors affecting DNA binding and stability of association to cationic liposomes. Chem. Phys. Lipids 2012, 165, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.C.; Hui, S.W. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther. 1999, 6, 651. [Google Scholar] [CrossRef] [PubMed]
- Rak, M.; Ochałek, A.; Bielecka, E.; Latasiewicz, J.; Gawarecka, K.; Sroka, J.; Czyż, J.; Piwowarczyk, K.; Masnyk, M.; Chmielewski, M.; et al. Efficient and non-toxic gene delivery by anionic lipoplexes based on polyprenyl ammonium salts and their effects on cell physiology. J. Gene Med. 2016, 18, 331–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oskuee, R.K.; Mahmoudi, A.; Gholami, L.; Rahmatkhah, A.; Malaekeh-Nikouei, B. Cationic Liposomes Modified with Polyallylamine as a Gene Carrier: Preparation, Characterization and Transfection Efficiency Evaluation. Adv. Pharm. Bull. 2016, 6, 515–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. In Protocols in In Vitro Hepatocyte Research; Vinken, M., Rogiers, V., Eds.; Springer: New York, NY, USA, 2015; pp. 333–348. [Google Scholar]
- Smale, S.T. Luciferase Assay. Cold Spring Harbor Protoc. 2010, 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Meidan, V.M.; Cohen, J.S.; Amariglio, N.; Hirsch-Lerner, D.; Barenholz, Y. Interaction of oligonucleotides with cationic lipids: The relationship between electrostatics, hydration and state of aggregation1. Biochim. Biophys. Acta Biomembr. 2000, 1464, 251–261. [Google Scholar] [CrossRef]
- Zuhorn, I.S.; Hoekstra, D. On the Mechanism of Cationic Amphiphile-mediated Transfection. To Fuse or not to Fuse: Is that the Question? J. Membr. Biol. 2002, 189, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, D.; Rejman, J.; Wasungu, L.; Shi, F.; Zuhorn, I. Gene delivery by cationic lipids: In and out of an endosome. Biochem. Soc. Trans. 2007, 35, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.B.; Calvin, S.A.; Lobenhofer, E.K. Transcriptional effects of transfection: The potential for misinterpretation of gene expression data generated from transiently transfected cells. BioTechniques 2009, 47, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, S.; Jiang, H.; Zhao, B.; Lv, H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Control. Release 2007, 123, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, N.; Kojima, C.; Harada, A.; Koiwai, K.; Kono, K. The correlation between fusion capability and transfection activity in hybrid complexes of lipoplexes and pH-sensitive liposomes. Biomaterials 2008, 29, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 30 April 2018).
Sample Availability: Samples of the compounds D19 and C12-Man-Q are available from the authors. |












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apsite, G.; Timofejeva, I.; Vezane, A.; Vigante, B.; Rucins, M.; Sobolev, A.; Plotniece, M.; Pajuste, K.; Kozlovska, T.; Plotniece, A. Synthesis and Comparative Evaluation of Novel Cationic Amphiphile C12-Man-Q as an Efficient DNA Delivery Agent In Vitro. Molecules 2018, 23, 1540. https://doi.org/10.3390/molecules23071540
Apsite G, Timofejeva I, Vezane A, Vigante B, Rucins M, Sobolev A, Plotniece M, Pajuste K, Kozlovska T, Plotniece A. Synthesis and Comparative Evaluation of Novel Cationic Amphiphile C12-Man-Q as an Efficient DNA Delivery Agent In Vitro. Molecules. 2018; 23(7):1540. https://doi.org/10.3390/molecules23071540
Chicago/Turabian StyleApsite, Gunita, Irena Timofejeva, Aleksandra Vezane, Brigita Vigante, Martins Rucins, Arkadij Sobolev, Mara Plotniece, Karlis Pajuste, Tatjana Kozlovska, and Aiva Plotniece. 2018. "Synthesis and Comparative Evaluation of Novel Cationic Amphiphile C12-Man-Q as an Efficient DNA Delivery Agent In Vitro" Molecules 23, no. 7: 1540. https://doi.org/10.3390/molecules23071540