Biomolecule-Functionalized Smart Polydiacetylene for Biomedical and Environmental Sensing
Abstract
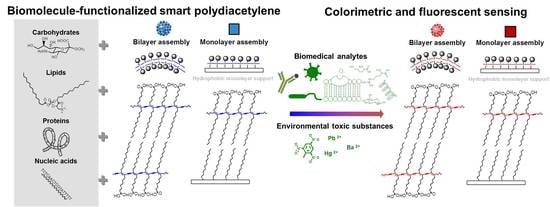
:1. Introduction
2. Carbohydrate-Functionalized Polydiacetylene (PDA)
3. Lipid-Functionalized PDA
4. Protein (Antibody) Functionalized PDA
5. Protein (Peptide or Amino Acid)-Functionalized PDA
6. Nucleic Acid Functionalized PDA
7. Other Biomolecule Functionalized PDAs
8. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okada, S.; Peng, S.; Spevak, W.; Charych, D. Color and chromism of polydiacetylene vesicles. Acc. Chem. Res. 1998, 31, 229–239. [Google Scholar] [CrossRef]
- UkáYe, B.; MináBaik, J.; HwaáKim, M. Unprecedented colorimetric responses of polydiacetylenes driven by plasma induced polymerization and their patterning applications. Chem. Commun. 2014, 50, 12447–12449. [Google Scholar]
- Lu, S.; Jia, C.; Duan, X.; Zhang, X.; Luo, F.; Han, Y.; Huang, H. Polydiacetylene vesicles for hydrogen peroxide detection. Colloids Surf. A 2014, 443, 488–491. [Google Scholar] [CrossRef]
- Sarkar, A.; Okada, S.; Matsuzawa, H.; Matsuda, H.; Nakanishi, H. Novel polydiacetylenes for optical materials: Beyond the conventional polydiacetylenes. J. Mater. Chem. 2000, 10, 819–828. [Google Scholar] [CrossRef]
- Ahn, D.J.; Kim, J.-M. Fluorogenic polydiacetylene supramolecules: Immobilization, micropatterning, and application to label-free chemosensors. Acc. Chem. Res. 2008, 41, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.-Y.; Chen, X.; Yoon, J. Recent progress in stimuli-induced polydiacetylenes for sensing temperature, chemical and biological targets. Chem. Commun. 2016, 52, 9178–9196. [Google Scholar] [CrossRef] [PubMed]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus-sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Kovbasnjuk, O. New insights into the role of Shiga toxins in intestinal disease. Gastroenterology 2005, 129, 1354–1355. [Google Scholar] [CrossRef] [PubMed]
- Nobile-Orazio, E.; Carpo, M.; Scarlato, G. Gangliosides. Drugs 1994, 47, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Goñi, F.M. The basic structure and dynamics of cell membranes: An update of the Singer–Nicolson model. Biochim. Biophys. Acta 2014, 1838, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Charych, D.H.; Nagy, J.O.; Spevak, W.; Bednarskit, M.D. Direct colorimetric detection of a receptor-ligand interaction by a polymerized bilayer assembly. Science 1993, 261, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Charych, D.H.; Spevak, W.; Nagy, J.O.; Bednarski, M.D. Specific Interaction of Influenza Virus with Organized Assemblies of Polydiacetylenes. MRS Online Proc. Libr. Arch. 1992, 292, 153. [Google Scholar] [CrossRef]
- Reichert, A.; Nagy, J.O.; Spevak, W.; Charych, D. Polydiacetylene liposomes functionalized with sialic acid bind and colorimetrically detect influenza virus. J. Am. Chem. Soc. 1995, 117, 829–830. [Google Scholar] [CrossRef]
- Lio, A.; Reichert, A.; Ahn, D.J.; Nagy, J.O.; Salmeron, M.; Charych, D.H. Molecular imaging of thermochromic carbohydrate-modified polydiacetylene thin films. Langmuir 1997, 13, 6524–6532. [Google Scholar] [CrossRef]
- Ma, B.; Fan, Y.; Zhang, L.; Kong, X.; Li, Y.; Li, J. Direct colorimetric study on the interaction of Escherichia coli with mannose in polydiacetylene Langmuir–Blodgett films. Colloids Surf. B 2003, 27, 209–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Y.; Sun, C.; Shen, D.; Li, Y.; Li, J. Functionalized polydiacetylene-glycolipid vesicles interacted with Escherichia coli under the TiO2 colloid. Colloids Surf. B 2005, 40, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.P.; Assali, M.; Fernández, I.; Khiar, N. Copper-Catalyzed Azide–Alkyne Cycloaddition in the Synthesis of Polydiacetylene: “Click Glycoliposome” as Biosensors for the Specific Detection of Lectins. Chemistry 2011, 17, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Jeong, D.; Cho, E.; Jung, S. Colorimetric detection of some highly hydrophobic flavonoids using polydiacetylene liposomes containing pentacosa-10, 12-diynoyl succinoglycan monomers. PLoS ONE 2015, 10, e0143454. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Cho, E.; Dindulkar, S.D.; Jung, S. Succinoglycan Octasaccharide Conjugated Polydiacetylene-Doped Alginate Beads for Barium (II) Detection. Macromol. Mater. Eng. 2016, 301, 805–811. [Google Scholar] [CrossRef]
- Cho, E.; Kim, H.; Choi, Y.; Paik, S.R.; Jung, S. Polydiacetylenyl β-cyclodextrin based smart vesicles for colorimetric assay of arginine and lysine. Sci. Rep. 2016, 6, 31115. [Google Scholar] [CrossRef] [PubMed]
- Charych, D.; Cheng, Q.; Reichert, A.; Kuziemko, G.; Stroh, M.; Nagy, J.O.; Spevak, W.; Stevens, R.C. A ‘litmus test’ for molecular recognition using artificial membranes. Chem. Biol. 1996, 3, 113–120. [Google Scholar] [CrossRef]
- Pan, J.J.; Charych, D. Molecular recognition and colorimetric detection of cholera toxin by poly (diacetylene) liposomes incorporating Gm1 ganglioside. Langmuir 1997, 13, 1365–1367. [Google Scholar] [CrossRef]
- Ma, Z.; Li, J.; Liu, M.; Cao, J.; Zou, Z.; Tu, J.; Jiang, L. Colorimetric detection of Escherichia coli by polydiacetylene vesicles functionalized with glycolipid. J. Am. Chem. Soc. 1998, 120, 12678–12679. [Google Scholar] [CrossRef]
- Ma, Z.; Li, J.; Jiang, L.; Cao, J.; Boullanger, P. Influence of the Spacer Length of Glycolipid Receptors in Polydiacetylene Vesicles on the Colorimetric Detection of Escherichia coli. Langmuir 2000, 16, 7801–7804. [Google Scholar] [CrossRef]
- Guo, C.X.; Boullanger, P.; Liu, T.; Jiang, L. Size effect of polydiacetylene vesicles functionalized with glycolipids on their colorimetric detection ability. J. Phys. Chem. B 2005, 109, 18765–18771. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Li, J.R.; Jiang, L.; Cao, J. Biosensor signal amplification of vesicles functionalized with glycolipid for colorimetric detection of Escherichia coli. J. Colloid Interface Sci. 2005, 284, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Stevens, R.C. Monolayer properties of monosialioganglioside in the mixed diacetylene lipid films on the air/water interface. Chem. Phys. Lipids 1997, 87, 41–53. [Google Scholar] [CrossRef]
- Deng, J.; Sheng, Z.; Zhou, K.; Duan, M.; Yu, C.-Y.; Jiang, L. Construction of effective receptor for recognition of avian influenza H5N1 protein HA1 by assembly of monohead glycolipids on polydiacetylene vesicle surface. Bioconjugate Chem. 2009, 20, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M.; Löwik, D.W.; van Hest, J.C. Effect of the diacetylene position on the chromatic properties of polydiacetylenes from self-assembled peptide amphiphiles. Biomacromolecules 2010, 11, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Schott, M. The colors of polydiacetylenes: A commentary. J. Phys. Chem. B 2006, 110, 15864–15868. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Lee, S.; Kim, J.-M. Recent conceptual and technological advances in polydiacetylene-based supramolecular chemosensors. Chem. Soc. Rev. 2009, 38, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, R.; Okada, S.; Norvez, S.; Charych, D. Interfacial catalysis by phospholipases at conjugated lipid vesicles: Colorimetric detection and NMR spectroscopy. Chem. Biol. 1998, 5, 619–629. [Google Scholar] [CrossRef]
- Okada, S.Y.; Jelinek, R.; Charych, D. Induced color change of conjugated polymeric vesicles by interfacial catalysis of phospholipase A2. Angew. Chem. Int. Ed. 1999, 38, 655–659. [Google Scholar] [CrossRef]
- Rozner, S.; Kolusheva, S.; Cohen, Z.; Dowhan, W.; Eichler, J.; Jelinek, R. Detection and analysis of membrane interactions by a biomimetic colorimetric lipid/polydiacetylene assay. Anal. Biochem. 2003, 319, 96–104. [Google Scholar] [CrossRef]
- Nie, Q.; Zhang, Y.; Zhang, J.; Zhang, M. Immobilization of polydiacetylene onto silica microbeads for colorimetric detection. J. Mater. Chem. 2006, 16, 546–549. [Google Scholar] [CrossRef]
- Kolusheva, S.; Shahal, T.; Jelinek, R. Peptide—Membrane interactions studied by a new phospholipid/polydiacetylene colorimetric vesicle assay. Biochemistry 2000, 39, 15851–15859. [Google Scholar] [CrossRef] [PubMed]
- Kolusheva, S.; Boyer, L.; Jelinek, R. A colorimetric assay for rapid screening of antimicrobial peptides. Nat. Biotechnol. 2000, 18, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-S.; Ahn, N.; Lee, J.; Seo, S.; Suh, K.-Y.; Kim, J.; Kim, K. Biomimetic detection of aminoglycosidic antibiotics using polydiacetylene–phospholipids supramolecules. Chem. Commun. 2012, 48, 5313–5315. [Google Scholar]
- Evrard, D.; Touitou, E.; Kolusheva, S.; Fishov, Y.; Jelinek, R. A new colorimetric assay for studying and rapid screening of membrane penetration enhancers. Pharm. Res. 2001, 18, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Sokolovski, M.; Sheynis, T.; Kolusheva, S.; Jelinek, R. Membrane interactions and lipid binding of casein oligomers and early aggregates. Biochim. Biophys. Acta 2008, 1778, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.P.; Soares, N.D.F.F.; Fontes, E.A.F.; de Oliveira, T.V.; Maradini Filho, A.M. Behaviour of polydiacetylene vesicles under different conditions of temperature, pH and chemical components of milk. Food Chem. 2012, 135, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Kolusheva, S.; Wachtel, E.; Jelinek, R. Biomimetic lipid/polymer colorimetric membranes molecular and cooperative properties. J. Lipid Res. 2003, 44, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Cheng, Q. Vesicular polydiacetylene sensor for colorimetric signaling of bacterial pore-forming toxin. Langmuir 2005, 21, 6123–6126. [Google Scholar] [CrossRef] [PubMed]
- Hanin-Avraham, N.; Fuhrman, B.; Mech-Dorosz, A.; Kolusheva, S.; Porgador, A.; Aviram, M.; Jelinek, R. Lipoprotein interactions with chromatic membranes as a novel marker for oxidative stress-related diseases. Biochim. Biophys. Acta 2009, 1788, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Volinsky, R.; Kliger, M.; Sheynis, T.; Kolusheva, S.; Jelinek, R. Glass-supported lipid/polydiacetylene films for colour sensing of membrane-active compounds. Biosens. Bioelectron. 2007, 22, 3247–3251. [Google Scholar] [CrossRef] [PubMed]
- Scindia, Y.; Silbert, L.; Volinsky, R.; Kolusheva, S.; Jelinek, R. Colorimetric detection and fingerprinting of bacteria by glass-supported lipid/polydiacetylene films. Langmuir 2007, 23, 4682–4687. [Google Scholar] [CrossRef] [PubMed]
- Kolusheva, S.; Yossef, R.; Kugel, A.; Katz, M.; Volinsky, R.; Welt, M.; Hadad, U.; Drory, V.; Kliger, M.; Rubin, E. Array-based disease diagnostics using lipid/polydiacetylene vesicles encapsulated in a sol–gel matrix. Anal. Chem. 2012, 84, 5925–5931. [Google Scholar] [CrossRef] [PubMed]
- Pevzner, A.; Kolusheva, S.; Orynbayeva, Z.; Jelinek, R. Giant chromatic lipid/polydiacetylene vesicles for detection and visualization of membrane interactions. Adv. Funct. Mater. 2008, 18, 242–247. [Google Scholar] [CrossRef]
- Peng, H.-P.; Lee, K.H.; Jian, J.-W.; Yang, A.-S. Origins of specificity and affinity in antibody–protein interactions. Proc. Natl. Acad. Sci. USA 2014, 111, E2656–E2665. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Li, J.-R.; Jiang, L. Chromatic immunoassay based on polydiacetylene vesicles. Colloids Surf. B 2004, 38, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Luo, J.; Dong, W.; Wang, C.; Jin, W.; Xia, Y.; Wang, H.; Ding, H.; Jiang, L.; He, H. Development and evaluation of a polydiacetylene based biosensor for the detection of H5 influenza virus. J. Virol. Methods 2015, 219, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-P.; Cho, E.; Yun, D.; Kim, T.; Lee, I.-S.; Jung, S. Label-Free Colorimetric Detection of Influenza Antigen Based on an Antibody-Polydiacetylene Conjugate and Its Coated Polyvinylidene Difluoride Membrane. Polymers 2017, 9, 127. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, J.P.; Lee, S.W.; Jeon, N.L.; Yoo, P.J.; Sim, S.J. A Direct, Multiplex Biosensor Platform for Pathogen Detection Based on Cross-linked Polydiacetylene (PDA) Supramolecules. Adv. Funct. Mater. 2009, 19, 3703–3710. [Google Scholar] [CrossRef]
- Kwon, I.K.; Kim, J.P.; Sim, S.J. Enhancement of sensitivity using hybrid stimulus for the diagnosis of prostate cancer based on polydiacetylene (PDA) supramolecules. Biosens. Bioelectron. 2010, 26, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Won, S.H.; Sim, S.J. Signal enhancement of a micro-arrayed polydiacetylene (PDA) biosensor using gold nanoparticles. Analyst 2012, 137, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.-C.; Shin, Y.-J.; Jeon, T.-J.; Kim, H.-Y.; Kim, Y.-R. Microbead-assisted PDA sensor for the detection of genetically modified organisms. Anal. Bioanal. Chem. 2011, 400, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-H.; Jang, H.; Lim, M.-C.; Kim, J.-H.; Shin, K.-S.; Kim, S.M.; Kim, H.-Y.; Kim, Y.-R.; Jeon, T.-J. Chromatic biosensor for detection of phosphinothricin acetyltransferase by use of polydiacetylene vesicles encapsulated within automatically generated immunohydrogel beads. Anal. Chem. 2015, 87, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Deng, J.; Jiang, L. Simple and highly sensitive detection of hepatotoxin microcystin-LR via colorimetric variation based on polydiacetylene vesicles. Sens. Actuators B 2010, 145, 713–719. [Google Scholar] [CrossRef]
- De Oliveira, T.V.; de FF Soares, N.; Coimbra, J.S.D.R.; de Andrade, N.J.; Moura, L.G.; Medeiros, E.A.; de Medeiros, H.S. Stability and sensitivity of polydiacetylene vesicles to detect Salmonella. Sens. Actuators B 2015, 221, 653–658. [Google Scholar] [CrossRef]
- Gill, I.; Ballesteros, A. Immunoglobulin–Polydiacetylene Sol–Gel Nanocomposites as Solid-State Chromatic Biosensors. Angew. Chem. 2003, 115, 3386–3389. [Google Scholar] [CrossRef]
- Lee, S.W.; Kang, C.D.; Yang, D.H.; Lee, J.S.; Kim, J.M.; Ahn, D.J.; Sim, S.J. The development of a generic bioanalytical matrix using polydiacetylenes. Adv. Funct. Mater. 2007, 17, 2038–2044. [Google Scholar] [CrossRef]
- Pindzola, B.A.; Nguyen, A.T.; Reppy, M.A. Antibody-functionalized polydiacetylene coatings on nanoporous membranes for microorganism detection. Chem. Commun. 2006, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Choi, H.; Lee, G.S.; Ahn, D.J.; Oh, M.-K. Effect of phospholipid insertion on arrayed polydiacetylene biosensors. Colloids Surf. B 2008, 66, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Kolusheva, S.; Kafri, R.; Katz, M.; Jelinek, R. Rapid Colorimetric Detection of Antibody—Epitope Recognition at a Biomimetic Membrane Interface. J. Am. Chem. Soc. 2001, 123, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Jung, H.-S.; Lee, J.; Seo, S.; Kim, J.; Kim, K.; Suh, K.-Y. Design of polydiacetylene-phospholipid supramolecules for enhanced stability and sensitivity. Langmuir 2012, 28, 7551–7556. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, J.; Choi, E.J.; Kim, E.J.; Song, J.Y.; Kim, J. Polydiacetylene Liposome Microarray Toward Influenza A Virus Detection: Effect of Target Size on Turn-On Signaling. Macromol. Rapid Commun. 2013, 34, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Onishi, A.; Saito, T.; Shimada, A.; Inoue, H.; Taki, T.; Nagata, K.; Okahata, Y.; Sato, T. Sialic acid-mimic peptides as hemagglutinin inhibitors for anti-influenza therapy. J. Med. Chem. 2010, 53, 4441–4449. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ha, K.; Guk, K.; Hwang, S.-G.; Choi, J.M.; Kang, T.; Bae, P.; Jung, J.; Lim, E.-K. Colorimetric detection of influenza A (H1N1) virus by a peptide-functionalized polydiacetylene (PEP-PDA) nanosensor. RSC Adv. 2016, 6, 48566–48570. [Google Scholar] [CrossRef]
- Cheng, Q.; Stevens, R.C. Coupling of an induced fit enzyme to polydiacetylene thin films: Colorimetric detection of glucose. Adv. Mater. 1997, 9, 481–483. [Google Scholar] [CrossRef]
- Kolusheva, S.; Shahal, T.; Jelinek, R. Cation-Selective Color Sensors Composed of Ionophore−Phospholipid−Polydiacetylene Mixed Vesicles. J. Am. Chem. Soc. 2000, 122, 776–780. [Google Scholar] [CrossRef]
- Jaworski, J.; Yokoyama, K.; Zueger, C.; Chung, W.-J.; Lee, S.-W.; Majumdar, A. Polydiacetylene incorporated with peptide receptors for the detection of trinitrotoluene explosives. Langmuir 2011, 27, 3180–3187. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.W.; Raorane, D.; Huh, J.H.; Majumdar, A.; Lee, S.-W. Evolutionary screening of biomimetic coatings for selective detection of explosives. Langmuir 2008, 24, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, B.Y.; Jaworski, J.; Yokoyama, K.; Chung, W.-J.; Wang, E.; Hong, S.; Majumdar, A.; Lee, S.-W. Selective and sensitive TNT sensors using biomimetic polydiacetylene-coated CNT-FETs. ACS Nano 2011, 5, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zawistowski, A.; Ehrmann, M.; Yi, T.; Schmuck, C. Peptide functionalized polydiacetylene liposomes act as a fluorescent turn-on sensor for bacterial lipopolysaccharide. J. Am. Chem. Soc. 2011, 133, 9720–9723. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, X.Y.; Schlesiger, S.; Li, M.; Zellermann, E.; Knauer, S.K.; Schmuck, C. Morphology-Dependent Cell Imaging by Using a Self-Assembled Diacetylene Peptide Amphiphile. Angew. Chem. 2017. [Google Scholar] [CrossRef]
- Rangin, M.; Basu, A. Lipopolysaccharide identification with functionalized polydiacetylene liposome sensors. J. Am. Chem. Soc. 2004, 126, 5038–5039. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Drickamer, K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Wen, Y.; Ding, B.; Sun, G.; Ke, T.; Chen, J.; Yu, J. Constitution of a visual detection system for lead (II) on polydiacetylene–glycine embedded nanofibrous membranes. J. Mater. Chem. A 2015, 3, 9722–9730. [Google Scholar] [CrossRef]
- Ripoll, M.; Neuberg, P.; Kichler, A.; Tounsi, N.; Wagner, A.; Remy, J.-S. PH-responsive nanometric polydiacetylenic micelles allow for efficient intracellular siRNA delivery. ACS Appl. Mater. Interfaces 2016, 8, 30665–30670. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, Z. Colorimetric detection of oligonucleotides using a polydiacetylene vesicle sensor. Anal. Bioanal. Chem. 2005, 382, 1708–1710. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-K.; Kim, K.-W.; Ahn, D.J.; Oh, M.-K. Label-free detection of bacterial RNA using polydiacetylene-based biochip. Biosens. Bioelectron. 2012, 35, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, J.A.; Burnett, J.F. A New Model for the K+-Induced Macromolecular Structure of Guanosine 5′-Monophosphate in Solution. Biochemistry 1999, 38, 14063–14068. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.-J.; Kim, J. Polydiacetylene liposome arrays for selective potassium detection. J. Am. Chem. Soc. 2008, 130, 5010–5011. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jun, H.; Kim, J. Polydiacetylene–liposome microarrays for selective and sensitive mercury (II) detection. Adv. Mater. 2009, 21, 3674–3677. [Google Scholar] [CrossRef]
- Jung, Y.K.; Kim, T.W.; Park, H.G.; Soh, H.T. Specific Colorimetric Detection of Proteins Using Bidentate Aptamer-Conjugated Polydiacetylene (PDA) Liposomes. Adv. Funct. Mater. 2010, 20, 3092–3097. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.; Zheng, M.; Zhong, Y.; Yang, J.; Zhao, Y.; Wu, W.; Ye, W.; Wen, J.; Wang, Q. An aptamer-based biosensor for colorimetric detection of Escherichia coli O157:H7. PLoS ONE 2012, 7, e48999. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.T.; Bohorquez, K.; Tsutsui, H. Polydiacetylene-coated polyvinylidene fluoride strip aptasensor for colorimetric detection of zinc (II). Sens. Actuators B 2016, 232, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Wilchek, M. [14] Protein biotinylation. Methods Enzymol. 1990, 184, 138–160. [Google Scholar] [PubMed]
- Jung, Y.K.; Park, H.G.; Kim, J.-M. Polydiacetylene (PDA)-based colorimetric detection of biotin–streptavidin interactions. Biosens. Bioelectron. 2006, 21, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.K.; Kim, T.W.; Jung, C.; Cho, D.Y.; Park, H.G. A polydiacetylene microchip based on a biotin–streptavidin interaction for the diagnosis of pathogen infections. Small 2008, 4, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; An, X.; Yan, X. Folate-polydiacetylene-liposome for tumor targeted drug delivery and fluorescent tracing. Colloids Surf. B 2015, 134, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R.; Kamen, B.A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar] [PubMed]
- Wang, D.-E.; Wang, Y.; Tian, C.; Zhang, L.; Han, X.; Tu, Q.; Yuan, M.; Chen, S.; Wang, J. Polydiacetylene liposome-encapsulated alginate hydrogel beads for Pb2+ detection with enhanced sensitivity. J. Mater. Chem. A 2015, 3, 21690–21698. [Google Scholar] [CrossRef]
- Ma, X.; Sheng, Z.; Jiang, L. Sensitive naked-eye detection of Hg2+ based on the aggregation and filtration of thymine functionalized vesicles caused by selective interaction between thymine and Hg2+. Analyst 2014, 139, 3365–3368. [Google Scholar] [CrossRef] [PubMed]




| Active Component | Analytes | Chemical Structures | Ref |
|---|---|---|---|
| Sialic acid | virus |  | [11,12,13,14,21,27] |
| Mannose | E. coli |  | [15] |
| E. coli |  | [16] | |
| lectin |  | [17] | |
| Succinoglycan octasaccharide | flavonoids |  | [18] |
| Ba2+ |  | [19] | |
| β-cyclodextrin | arginine, lysine |  | [20] |
| GM1 gaglioiside | cholera toxin |  | [21,22,27] |
| GTb1 gaglioiside | botulinum neurotoxin |  | [21] |
| β-glucoside | E. coli |  | [23] |
| E. coli |  | [24] | |
| N-acetamide-β-glucoside | lectin |  | [25] |
| β-maltotriose | E. coli |  | [26] |
| Sialic acid-β-glucoside | hemagglutinin (HA1) |  | [28] |
| Biomimetic PDA | Detection Fields | Ref |
|---|---|---|
| DMPC 1/PDA | Phospholipase action | [32,33,34,35] |
| Penetration enhancers (azone, oleic acid etc.) | [39] | |
| Casein oligomers | [40] | |
| α-Lactoalbumin, β-lactoglobulin | [41] | |
| DMPE 2, DMPC/PDA | Antimicrobial peptides (mellitin, maganin, alamethicin, polymixin B) | [36,37,45] |
| DMPC,sphingomyelin,cholesterol/PDA | Polymixin B | [45] |
| Bacterial fingerprinting | [46] | |
| DMPC,DMPE,DGS 3,DMPG 4/PDA | Lipoproteins | [44] |
| Plasma molecular components | [47] | |
| DMPC, DMPE, DMPG/PDA giant vesicles | Vaccina virus, polymyxin B, lipophilic pharmaceutical (nortriptyline) | [48] |
| PIP2 5/PDA | Aminoglycoside antibiotics (neomycin) | [38] |
| Sphingomyelin/PDA | Sphingomyelinase action | [34] |
| Cerebroside/PDA | Galactosidase action | [34] |
| Cholesterol/PDA | SLO 6 | [43] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Jung, S. Biomolecule-Functionalized Smart Polydiacetylene for Biomedical and Environmental Sensing. Molecules 2018, 23, 107. https://doi.org/10.3390/molecules23010107
Cho E, Jung S. Biomolecule-Functionalized Smart Polydiacetylene for Biomedical and Environmental Sensing. Molecules. 2018; 23(1):107. https://doi.org/10.3390/molecules23010107
Chicago/Turabian StyleCho, Eunae, and Seunho Jung. 2018. "Biomolecule-Functionalized Smart Polydiacetylene for Biomedical and Environmental Sensing" Molecules 23, no. 1: 107. https://doi.org/10.3390/molecules23010107