Comparative Analysis of Amaryllidaceae Alkaloids from Three Lycoris Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic Conditions
2.2. Sample Analysis and Amaryllidaceae Alkaloids’ (AAs) Identification
2.2.1. HPLC Fingerprint Profiles

2.2.2. Identification of AAs

| Peak No. | Rt (min) | PI-MS [M + H]+ | MS/MS Data | Identification | Alkaloid Type/Ref. |
|---|---|---|---|---|---|
| 1 | 3.22 | 284 | 266, 256, 249, 241, 237, 226, 213, 199 | 1,11-didehydrogalanthamine b | GA [17] |
| 2 | 3.61 | 304 | 286, 268, 250, 227, 211, 150 | 11-hydroxyvittatine N-oxide a | CR [27,33] |
| 3 | 3.93 | 290 | 272, 225, 217, 211, 201, 199, 181, 124, 119 | 3-hydroxylatifaliumin C b | TA [33] |
| 4 | 4.97 | 276 | 258, 219, 215, 201, 189, 175, 153 | Dihydro-latifaliuminC a | TA [33] |
| 5 | 5.71 | 274 | 256, 225, 217, 207, 199, 197, 181 | Latifaliumin C a | TA [33] |
| 6 | 5.76 | 350 | 332, 281, 267, 255, 223, 193, 180 | Unidentified | |
| 7 | 6.54 | 334 | 316, 298, 270, 267, 255, 238, 173 | 2α-Hydroxy-3-hydro-6-O-methyloduline b | HO [36] |
| 8 | 7.17 | 274 | 231, 225, 213, 198, 183 | N-demethyl-galanthamine | GA [26,27] |
| 9 | 9.48 | 290 | 272, 254, 226, 149, 136, 112, 68 | Dihydrolycorine | LY [29,30,31] |
| 10 | 10.05 | 306 | 288, 270, 229, 189 | Crinamabine a | CR [35] |
| 11 | 12.90 | 288 | 270, 252, 222, 177, 147, 119, 95 | Lycorine | LY [1,17] |
| 12 | 14.08 | 286 | 250, 240, 226, 147 | (+)-5,6-dehydrolycorine | LY [5] |
| 13 | 14.79 | 290 | 272, 233, 215, 189 | lycoramine | GA [1] |
| 14 | 17.40 | 288 | 270, 231, 225, 213, 198, 181 | Galanthamine | GA [17] |
| 15 | 18.24 | 306 | 288, 247, 233, 229, 215, 201, 189 | lycoramine N-oxide | GA [15,25] |
| 16 | 22.04 | 264 | 247, 189, 166, 149, 133, 116 | Hippadine | LY [32] |
| 17 | 23.23 | 288 | 270, 255, 239, 193, 162, 151, 121, 108, 94 | Pluviine | LY [16,19] |
| 18 | 24.20 | 262 | 244, 228, 219, 205, 179, 165, 147, 123, 98, 91 | Unidentified | |
| 19 | 26.58 | 302 | 284, 266, 255, 193, 175, 145, 108, 94 | Oduline | HO [36] |
| 20 | 26.74 | 332 | 300, 282, 264, 234, 225, 213, 199, 169 | Ambelline a | CR [17,34] |
| 21 | 27.33 | 272 | 254, 242, 226, 149, 136, 108 | Vittatine | CR [17] |
| 22 | 27.88 | 318 | 300, 286, 268, 250, 227, 209, 199, 149 | Crinamidine a | CR [17,33] |
| 23 | 29.08 | 332 | 300, 282, 275, 267, 255, 243, 223, 195, 124 | Hippeastrine N-oxide | HO [37] |
| 24 | 29.15 | 286 | 255, 229, 225, 197, 179, 168, 58 | Narwedine a | GA [28] |
| 25 | 29.64 | 302 | 270, 259, 226, 211, 196, 181, 168 | Haemanthamine | CR [17] |
| 26 | 30.96 | 316 | 298, 280, 273, 239, 222, 191 ,126, 96, 83 | hippeastrine | HO [36] |
| 27 | 31.86 | 332 | 314, 282, 253, 239, 211, 223, 175, 96 | 2α-Hydroxy-6-O-methyloduline | HO [36] |
| 28 | 32.82 | 316 | 298, 280, 267, 239, 207, 191, 176, 160, 108, 94 | (+)-8,9-methylenedioxylhomolycorine N-oxide | HO [5] |
| 29 | 33.15 | 318 | 286, 271, 267, 177 | Unidentified | |
| 30 | 33.38 | 332 | 300, 282, 257, 251, 243, 191, 163, 94, | 2-methoxyoduline b | HO [36] |
| 31 | 34.49 | 334 | 302, 270, 259, 245, 231, 217, 213, 199 | 3,11-dimethoxy-lycoramine b | GA [17] |
| 32 | 35.58 | 316 | 285, 267, 256, 239, 228, 207, 175, 157, 129, 118 | Unidentified | |
| 33 | 36.19 | 332 | 300, 284, 271, 251, 239, 219, 191, 94, 81 | Unidentified | |
| 34 | 36.19 | 266 | 250, 236, 222, 208, 109 | Unidentified | |
| 35 | 36.84 | 332 | 314, 300, 282, 271, 264, 240, 224, 211, 181, 153, 120, 107 | Unidentified | |
| 36 | 37.56 | 346 | 314, 282, 253, 239, 225, 211, 175, 147, 96 | 2α-Methoxy-6-O-methyloduline | HO [36] |
| 37 | 40.20 | 344 | 312, 280, 266, 252, 195, 89 | Unidentified | |
| 38 | 41.15 | 298 | 270, 248, 238, 212, 180 | Unidentified | |
| 39 | 44.57 | 346 | 288, 241, 239, 211, 209, 183, 168, 140, 116, 94 | Unidentified |
2.2.3. Identification of Galanthamine Type Alkaloids

2.2.4. Identification of Lycorine Type Alkaloids
2.2.5. Identification of Crinine Type Alkaloids
2.2.6. Identification of Homolycorine Type Alkaloids

2.2.7. Identification of Tazettine Type Alkaloids

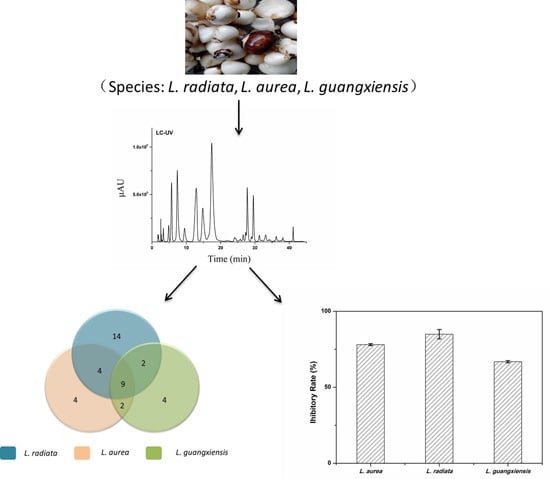
2.3. Comparison of AAs among the Three Lycoris Species

2.4. Comparison of Anti-HepG2 Activity of AAs in the Three Lycoris Species

3. Experimental Section
3.1. Chemicals
3.2. Plant Materials and Sample Preparation
3.3. HPLC-UV/ESI-MS/MS Analysis
3.3.1. HPLC-UV Conditions
3.3.2. ESI-MS/MS Conditions
3.4. Quantitative Analysis of AAs
3.5. Anti-HepG2 Activity Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, X.; Liu, Y.B.; Huang, S.; Liu, Y. An LC-MS/MS method for the simultaneous determination of lycorine and galanthamine in rat plasma and its application to pharmacokinetic study of Lycoris radiata extract in rats. J. Huazhong Univ. Sci. Technol. Med. 2014, 34, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. 2013, 62, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Zhang, L.; Song, Y. Alkaloids from Lycoris aurea and their cytotoxicities against the head and neck squamous cell carcinoma. Fitoterapia 2014, 95, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, C.Y.; Liu, S.L.; Wei, L.X. Structure investigation of a new alkaloid from Zanthoxylum schinifolium Siebet zucc. Chem. Res. Chin. Univ. 1991, 7, 124–128. [Google Scholar]
- Hao, B.; Shen, S.F.; Zhao, Q.J. Cytotoxic and antimalarial Amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Molecules 2013, 18, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Pan, D.S.; Han, S.; Yu, C.Y.; Zhao, Q.J.; Song, Y.; Liang, Y. Alkaloids from Lycoris caldwellii and their particular cytotoxicities against the astrocytoma and glioma cell lines. Arch. Pharm. Res. 2013, 36, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, M.; Stavrakov, G.; Philipova, I.; Zheleva, D.; Yordanov, N.; Doytchinova, I. Galantamine derivatives with indole moiety: Docking, design, synthesis and acetylcholinesterase inhibitory activity. Bioorg. Med. Chem. 2015, 23, 5382–5389. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, W.X.; He, L.F.; Ye, M.; Li, Y. Eeffectes of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004, 578, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, L.; Lefranc, F.; Mathieu, V.; Darro, F.; Kiss, R. Amaryllidaceae isocarbostyril alkaloids and their derivatives as promising antitumor agents. Transl. Oncol. 2008, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed]
- Lamoral-Theys, D.; Andolfi, A.; van Goietsenoven, G.; Cimmino, A.; le Calve, B.; Wauthoz, N.; Megalizzi, V.; Gras, T.; Bruyere, C.; Dubois, J.; et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure-activity relationship and mechanistic insight. J. Med. Chem. 2009, 52, 6244–6256. [Google Scholar] [CrossRef] [PubMed]
- Van Goietsenoven, G.; Mathieu, V.; Lefranc, F.; Kornienko, A.; Evidente, A.; Kiss, R. Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers. Med. Res. Rev. 2013, 33, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Sauvage, S.; van Goietsenoven, G.; Megalizzi, V.; Lamoral-Theys, D.; Debeir, O.; Spiegl-Kreinecker, S.; Berger, W.; Mathieu, V.; Decaestecker, C.; et al. Narciclasine, a plant growth modulator, activates rho and stress fibers in glioblastoma cells. Mol. Cancer Ther. 2009, 8, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Van Goietsenoven, G.; Hutton, J.; Becker, J.P.; Lallemand, B.; Robert, F.; Lefranc, F.; Pirker, C.; Vandenbussche, G.; van Antwerpen, P.; Evidente, A.; et al. Targeting of Eff1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas. FASEB J. 2010, 24, 4575–4584. [Google Scholar] [CrossRef] [PubMed]
- Giordani, R.B.; de Andrade, J.P.; Verli, H.; Dutilh, J.H.; Henriques, A.T.; Berkov, S.; Bastida, J.; Zuanazzi, J.A. Alkaloids from Hippeastrum morelianum Lem. (Amaryllidaceae). Magn. Reson. Chem. 2011, 49, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.M.; Zhu, Y.Y.; Li, H.R.; Yu, H.Y.; Zhang, P.; Pi, H.F.; Ruan, H.L. Two new alkaloids from the bulbs of Lycoris sprengeri. J. Asian Nat. Prod. Res. 2014, 16, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Katoch, D.; Kumar, S.; Kumar, N.; Singh, B. Simultaneous quantification of Amaryllidaceae alkaloids from Zephyranthes grandiflora by UPLC-DAD/ESI-MS/MS. J. Pharm. Biomed. Anal. 2012, 71, 187–192. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, J.P.; Pigni, N.B.; Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Bioactive alkaloid extracts from Narcissus broussonetii: Mass spectral studies. J. Pharm. Biomed. Anal. 2012, 70, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt-Sarikaya, B.; Kaya, G.; Onur, M.; Bastida, J.; Berkov, S.; Unver-Somer, N. GC/MS analysis of Amaryllidaceae alkaloids in Galanthus gracilis. Chem. Nat. Compd. 2014, 50, 573–575. [Google Scholar] [CrossRef]
- Berkov, S.; Bastida, J.; Viladomat, F.; Codina, C. Analysis of galanthamine-type alkaloids by capillary gas chromatography-mass spectrometry in plants. Phytochem. Anal. 2008, 19, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, T. Highly efficient, selective and sensitive molecular screening of acetylcholinesterase inhibitors of natural origin by solid-phase extraction-liquid chromatography/electrospray ionisation-octopole-orthogonal acceleration time-of-flight-mass spectrometry and novel thin-layer chromatography-based bioautography. J. Chromatogr. A 2009, 1216, 2519–2528. [Google Scholar] [PubMed]
- Zhang, Y.; Chen, Z. Nonaqueous CE ESI-IT-MS analysis of Amaryllidaceae alkaloids. J. Sep. Sci. 2013, 36, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Viladomat, F.; Codina, C.; Suarez, S.; Ravelo, A.; Bastida, J. GC-MS of Amaryllidaceous galanthamine-type alkaloids. J. Mass Spectrom. 2012, 47, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.D.; Zhang, Y.; He, H.P.; Li, S.F.; Tang, G.H.; Chen, D.Z.; Cao, M.M.; Di, Y.T.; Hao, X.J. A new Amaryllidaceae alkaloid from the bulbs of Lycoris radiata. Chin. J. Nat. Med. 2013, 11, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.F.; Zhang, P.; Ruan, H.L.; Zhang, Y.H.; Sun, H.D.; Wu, J.Z. A new alkaloid from Lycoris aurea. Chin. Chem. Lett. 2009, 20, 1319–1320. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, S.X.; Zhao, Y.M.; Zhao, Q.S.; Sun, H.D. Alkaloids from the bulbs of Lycoris aurea. Helv. Chim. Acta 2005, 88, 2550–2553. [Google Scholar] [CrossRef]
- Elgorashi, E.E.; Malan, S.F.; Stafford, G.I.; van Staden, J. Quantitative structure–activity relationship studies on acetylcholinesterase enzyme inhibitory effects of Amaryllidaceae alkaloids. S. Afr. J. Bot. 2006, 72, 224–231. [Google Scholar] [CrossRef]
- De Andrade, J.P.; Guo, Y.; Font-Bardia, M.; Calvet, T.; Dutilh, J.; Viladomat, F.; Codina, C.; Nair, J.J.; Zuanazzi, J.A.; Bastida, J. Crinine-type alkaloids from Hippeastrum aulicum and H. Calyptratum. Phytochemistry 2014, 103, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.Y.; Wang, Z.Y.; Pi, H.F.; Zhang, P.; Ruan, H.L. Neuroprotective compounds from the bulbs of Lycoris radiata. Fitoterapia 2013, 88C, 82–90. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qi, W.B.; Wang, L.; Tian, J.; Jiao, P.R.; Liu, G.Q.; Ye, W.C.; Liao, M. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir. Viruses 2013, 7, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, Y.Y.; Su, J.; Li, Y.; Cai, X.H.; Luo, X.D. Amaryllidaceae alkaloids from Lycoris radiata. Helv. Chim. Acta 2011, 94, 178–183. [Google Scholar] [CrossRef]
- Sun, Q.; Shen, Y.H.; Tian, J.M.; Tang, J.; Su, J.; Liu, R.H.; Li, H.L.; Xu, X.K.; Zhang, W.D. Chemical constituents of Crinum asiaticum L. Var. Sinicumbaker and their cytotoxic activities. Chem. Biodivers. 2009, 6, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, H.; Liang, X.; Huang, H.; Dai, W.; Shen, Y.; Yan, S.; Zhang, W. Analysis of Amaryllidaceae alkaloids from Crinum by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; Nair, J.J.; Codina, C.; Bastida, J.; Pandey, S.; Gerasimoff, J.; Griffin, C. Selective apoptosis-inducing activity of crinum-type Amaryllidaceae alkaloids. Phytochemistry 2007, 68, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Ao, M.; Zhang, P.; Yu, L. Extracts of Lycoris aurea induce apoptosis in murine sarcoma s180 cells. Molecules 2012, 17, 3723–3735. [Google Scholar] [CrossRef] [PubMed]
- Kihara, M.; Konishi, K.; Xu, L.; Kobayashi, S. Alkaloidal constituents of the flowers of Lycoris radiata herb (amaryllidaceae). Chem. Pharm. Bull. 1991, 39, 1849–1853. [Google Scholar] [CrossRef]
- Van Goietsenoven, G.; Andolfi, A.; Lallemand, B.; Cimmino, A.; Lamoral-Theys, D.; Gras, T.; Abou-Donia, A.; Dubois, J.; Lefranc, F.; Mathieu, V.; et al. Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cell. J. Nat. Prod. 2010, 73, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.H.; Döpke, W.; Wagner, J.; Mügge, C. Alkaloids from Crinum amabile. Phytochemistry 1998, 48, 371–376. [Google Scholar] [CrossRef]
- Liu, H.; Luan, F.; Ju, Y.; Shen, H.; Gao, L.; Wang, X.; Liu, S.; Zhang, L.; Sun, W.; Ma, C. In vitro transfection of the hepatitis Bvirus PreS2 gene into the human hepatocarcinoma cell line HepG2 induces upregulation of human telomerase reverse transcriptase. Biochem. Biophys. Res. Commun. 2007, 355, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.R.; Li, Q.; Liu, Z.L.; Liu, H.H.; Wang, W.; Liao, X.L.; Pan, Y.L.; Jiang, J.W. Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 599–604. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zhang, C.; Guo, M. Comparative Analysis of Amaryllidaceae Alkaloids from Three Lycoris Species. Molecules 2015, 20, 21854-21869. https://doi.org/10.3390/molecules201219806
Tian Y, Zhang C, Guo M. Comparative Analysis of Amaryllidaceae Alkaloids from Three Lycoris Species. Molecules. 2015; 20(12):21854-21869. https://doi.org/10.3390/molecules201219806
Chicago/Turabian StyleTian, Yongqiang, Chunyun Zhang, and Mingquan Guo. 2015. "Comparative Analysis of Amaryllidaceae Alkaloids from Three Lycoris Species" Molecules 20, no. 12: 21854-21869. https://doi.org/10.3390/molecules201219806