Electrochemistry of the Self-Assembled Monolayers of Dyads Consisting of Tripod-Shaped Trithiol and Bithiophene on Gold
Abstract
:1. Introduction


2. Results and Discussion
2.1. Synthesis of Tripodal Trithiol–Bithiophene Dyads

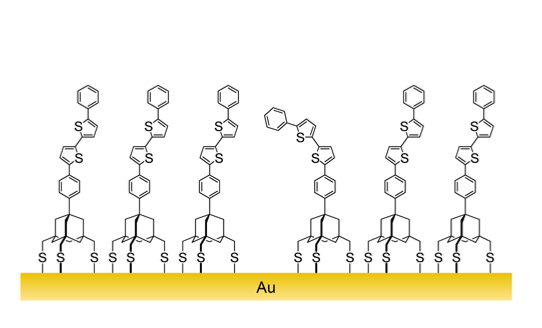
2.2. Formation of the SAM of 3 and Characterization by PM-IRRAS

2.3. Electrochemistry of the SAM of 3
2.3.1. Reductive Desorption

| Thiol | Ep | Qred b | ΔEfwhm |
|---|---|---|---|
| (V vs. Ag/AgCl) | (µC·cm−2) | (mV) | |
| 1 c | −1.088 | 100 | 91 |
| 2 d | −0.977 | 91 | 125 |
| 3a | −1.057 | 51 | 108 |
| 3b | −1.085 | 49 | 121 |
| n-C12H25SH c | −1.084 | 100 | 20 |

2.3.2. Oxidation of the Bithiophene Moiety


3. Experimental Section
3.1. General
3.2. Synthesis of 1-[4-(2,2′-Bithiophen-5-yl)phenyl]-3,5,7-tris(mercaptomethyl)adamantane (3a)
3.3. Synthesis of 1-[4-(5′-Phenyl-2,2′-bithiophen-5-yl)phenyl]-3,5,7-tris(mercaptomethyl)adamantane (3b)
3.4. Preparation of the Self-Assembled Monolayer on a Au(111) Substrate
3.5. Cyclic Voltammetry
3.6. Theoretical Calculations
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar]
- Poirier, G.E. Characterization of Organosulfur Molecular Monolayers on Au(111) using Scanning Tunneling Microscopy. Chem. Rev. 1997, 97, 1117–1127. [Google Scholar]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar]
- Kind, M.; Wöll, C. Organic surfaces exposed by self-assembled organothiol monolayers: Preparation, characterization, and application. Prog. Surf. Sci. 2009, 84, 230–278. [Google Scholar]
- Chaki, N.K.; Vijayamohanan, K. Self-assembled monolayers as a tunable platform for biosensor applications. Biosens. Bioelectron. 2002, 17, 1–12. [Google Scholar]
- Gooding, J.J.; Mearns, F.; Yang, W.; Liu, J. Self-Assembled Monolayers into the 21st Century: Recent Advances and Applications. Electroanalysis 2003, 15, 81–96. [Google Scholar]
- Bertin, P.A.; Ahrens, M.J.; Bhavsar, K.; Georganopoulou, D.; Wunder, M.; Blackburn, G.F.; Meade, T.J. Ferrocene and Maleimide-Functionalized Disulfide Scaffolds for Self-Assembled Monolayers on Gold. Org. Lett. 2010, 12, 3372–3375. [Google Scholar]
- Jian, H.; Tour, J.M. En Route to Surface-Bound Electric Field-Driven Molecular Motors. J. Org. Chem. 2003, 68, 5091–5103. [Google Scholar]
- Zheng, X.; Mulcahy, M.E.; Horinek, D.; Galeotti, F.; Magnera, T.F.; Michl, J. Dipolar and Nonpolar Altitudinal Molecular Rotors Mounted on an Au(111) Surface. J. Am. Chem. Soc. 2004, 126, 4540–4542. [Google Scholar]
- Van Delden, R.A.; ter Wiel, M.K.J.; Pollard, M.M.; Vicario, J.; Koumura, N.; Feringa, B.L. Unidirectional molecular motor on a gold surface. Nature 2005, 437, 1337–1340. [Google Scholar]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic Molecular Motors and Mechanical Machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar]
- Tour, J.M. Molecular Electronics. Synthesis and Testing of Components. Acc. Chem. Res. 2000, 33, 791–804. [Google Scholar]
- Donhauser, Z.J.; Mantooth, B.A.; Kelly, K.F.; Bumm, L.A.; Monnell, J.D.; Stapleton, J.J.; Price, D.W., Jr.; Rawlett, A.M.; Allara, D.L.; Tour, J.M.; et al. Conductance Switching in Single Molecules through Conformational Changes. Science 2001, 292, 2303–2307. [Google Scholar]
- Park, J.; Pasupathy, A.N.; Goldsmith, J.I.; Chang, C.; Yaish, Y.; Petta, J.R.; Rinkoski, M.; Sethna, J.P.; Abruña, H.D.; McEuen, P.L.; et al. Coulomb blockade and the Kondoeffect in single-atom transistors. Nature 2002, 417, 722–725. [Google Scholar]
- Lewis, P.A.; Inman, C.E.; Yao, Y.; Tour, J.M.; Hutchison, J.E.; Weiss, P.S. Mediating Stochastic Switching of Single Molecules Using Chemical Functionality. J. Am. Chem. Soc. 2004, 126, 12214–12215. [Google Scholar]
- McCreery, R.L. Molecular Electronic Junctions. Chem. Mater. 2004, 16, 4477–4496. [Google Scholar]
- Zhu, L.; Tang, H.; Harima, Y.; Yamashita, K.; Hirayama, D.; Aso, Y.; Otsubo, T. Electrochemical properties of self-assembled monolayers oftripod-shaped molecules and their applications to organiclight-emitting diodes. Chem. Commun. 2001, 1830–1831. [Google Scholar]
- Hirayama, D.; Takimiya, K.; Aso, Y.; Otsubo, T.; Hasobe, T.; Yamada, H.; Imahori, H.; Fukuzumi, S.; Sakata, Y. Large Photocurrent Generation of Gold Electrodes Modified with [60]Fullerene-Linked Oligothiophenes Bearing a Tripodal Rigid Anchor. J. Am. Chem. Soc. 2002, 124, 532–533. [Google Scholar]
- Zhu, L.; Tang, H.; Harima, Y.; Yamashita, K.; Aso, Y.; Otsubo, T. Enhanced hole injection in organic light-emitting diodes consisting of self-assembled monolayer of tripod-shaped π-conjugated thiols. J. Mater. Chem. 2002, 12, 2250–2254. [Google Scholar]
- Otsubo, T.; Aso, Y.; Takimiya, K. Functional oligothiophenes as advanced molecular electronic materials. J. Mater. Chem. 2002, 12, 2565–2575. [Google Scholar]
- Ie, Y.; Hirose, T.; Nakamura, H.; Kiguchi, M.; Takagi, N.; Kawai, M.; Aso, Y. Nature of Electron Transport by Pyridine-Based Tripodal Anchors: Potential for Robust and Conductive Single-Molecule Junctions with Gold Electrodes. J. Am. Chem. Soc. 2011, 133, 3014–3022. [Google Scholar]
- Hirose, T.; Ie, Y.; Aso, Y. Synthesis of Tripodal-anchor Units Having Pyridine or Amine Functional Groupsand Their Adsorption Behavior on Metal Electrodes. Chem. Lett. 2011, 40, 204–205. [Google Scholar]
- Kitagawa, T.; Idomoto, Y.; Matsubara, H.; Hobara, D.; Kakiuchi, T.; Okazaki, T.; Komatsu, K. Rigid Molecular Tripod with an Adamantane Framework and Thiol Legs. Synthesis and Observation of an Ordered Monolayer on Au(111). J. Org. Chem. 2006, 71, 1362–1369. [Google Scholar]
- Katano, S.; Kim, Y.; Matsubara, H.; Kitagawa, T.; Kawai, M. Hierarchical Chiral Framework Based on a Rigid Adamantane Tripod on Au(111). J. Am. Chem. Soc. 2007, 129, 2511–2515. [Google Scholar]
- Katano, S.; Kim, Y.; Kitagawa, T.; Kawai, M. Self-Assembly and Scanning Tunneling Microscopy Tip-Induced Motion of Ferrocene Adamantane Trithiolate Adsorbed onAu(111). Jpn. J. Appl. Phys. 2008, 47, 6156–6159. [Google Scholar]
- Kitagawa, T.; Matsubara, H.; Komatsu, K.; Hirai, K.; Okazaki, T.; Hase, T. Ideal Redox Behavior of the High-Density Self-Assembled Monolayer of a Molecular Tripod on a Au(111) Surface with a Terminal Ferrocene Group. Langmuir 2013, 29, 4275–4282. [Google Scholar]
- Katano, S.; Kim, Y.; Kitagawa, T.; Kawai, M. Tailoring electronic states of a single molecule using adamantane-based molecular tripods. Phys. Chem. Chem. Phys. 2013, 15, 14229–14233. [Google Scholar]
- Fox, M.A.; Whitesell, J.K.; McKerrow, A.J. Fluorescence and Redox Activity of Probes Anchored through an Aminotrithiol to Polycrystalline Gold. Langmuir 1998, 14, 816–820. [Google Scholar]
- Kittredge, K.W.; Minton, M.A.; Fox, M.A.; Whitesell, J.K. α-Helical Polypeptide Films Grown From Sulfide or Thiol Linkers on Gold Surfaces. Helv. Chim. Acta 2002, 85, 788–798. [Google Scholar]
- Wei, L.; Padmaja, K.; Youngblood, W.J.; Lysenko, A.B.; Lindsey, J.S.; Bocian, D.F. Diverse Redox-Active Molecules Bearing Identical Thiol-Terminated Tripodal Tethers for Studies of Molecular Information Storage. J. Org. Chem. 2004, 69, 1461–1469. [Google Scholar]
- Wei, L.; Tiznado, H.; Liu, G.; Padmaja, K.; Lindsey, J.S.; Zaera, F.; Bocian, D.F. Adsorption Characteristics of Tripodal Thiol-Functionalized Porphyrins on Gold. J. Phys. Chem. B 2005, 109, 23963–23971. [Google Scholar]
- Weidner, T.; Zharnikov, M.; Hoßbach, J.; Castner, D.G.; Siemeling, U. Adamantane-Based Tripodal Thioether Ligands Functionalized with a Redox-Active Ferrocenyl Moiety for Self-Assembled Monolayers. J. Phys. Chem. C 2010, 114, 14975–14982. [Google Scholar]
- Singhana, B.; Rittikulsittichai, S.; Lee, T.R. Tridentate Adsorbates with Cyclohexyl Headgroups Assembled on Gold. Langmuir 2013, 29, 561–569. [Google Scholar]
- Singhana, B.; Jamison, A.C.; Hoang, J.; Lee, T.R. Self-Assembled Monolayer Films Derived from Tridentate Cyclohexyl Adsorbates with Alkyl Tailgroups of Increasing Chain Length. Langmuir 2013, 29, 14108–14116. [Google Scholar]
- Ng, M.K.; Lee, D.C.; Yu, L. Molecular Diodes Based on Conjugated Diblock Co-oligomers. J. Am. Chem. Soc. 2002, 124, 11862–11863. [Google Scholar]
- Martin, P.; Rocca, M.L.D.; Anthore, A.; Lafarge, P.; Lacroix, J.C. Organic Electrodes Based on Grafted Oligothiophene Units inUltrathin, Large-Area Molecular Junctions. J. Am. Chem. Soc. 2012, 134, 154–157. [Google Scholar]
- Wan, D.; Yuan, S.; Neoh, K.G.; Kang, E.T. Surface Functionalization of Copper via Oxidative Graft Polymerization of 2,2′-Bithiophene and Immobilization of Silver Nanoparticles for Combating Biocorrosion. ACS Appl. Mater. Interfaces 2010, 2, 1653–1662. [Google Scholar]
- Malenfant, P.R.L.; Jayaraman, M.; Fréchet, J.M.J. Dendrimer-Supported Oligothiophene Synthesis: Aliphatic Ether Dendrimers in the Preparation of Oligothiophenes with Minimal Substitution. Chem. Mater. 1999, 11, 3420–3422. [Google Scholar]
- Morin, J.F.; Leclerc, M. Syntheses of Conjugated Polymers Derivedfrom N-Alkyl-2,7-carbazoles. Macromolecules 2001, 34, 4680–4682. [Google Scholar]
- Idzik, K.R.; Frydel, J.; Beckert, R.; Ledwon, P.; Lapkowski, M.; Fasting, C.; Müller, C.; Licha, T. Synthesis and electrochemical properties of tetrathienyl-linked branched polymers with various aromatic cores. Electrochim. Acta 2012, 79, 154–161. [Google Scholar]
- Walczak, M.M.; Popenoe, D.D.; Deinhammer, R.S.; Lamp, B.D.; Chung, C.; Porter, M.D. Reductive Desorption of Alkanethiolate Monolayers at Gold: A Measure of Surface Coverage. Langmuir 1991, 7, 2687–2693. [Google Scholar]
- Widrig, C.A.; Chung, C.; Porter, M.D. The electrochemical desorption of n-alkanethiol monolayers from polycrystalline Au and Ag electrodes. J. Electroanal. Chem. 1991, 310, 335–359. [Google Scholar]
- Yang, D.F.; Wilde, C.P.; Morin, M. Studies of the Electrochemical Removal and Efficient Re-formation of a Monolayer of Hexadecanethiol Self-Assembled at an Au(111) Single Crystal in Aqueous Solutions. Langmuir 1997, 13, 243–249. [Google Scholar]
- Imabayashi, S.; Iida, M.; Hobara, D.; Feng, Z.Q.; Niki, K.; Kakiuchi, T. Reductive desorption of carboxylic-acid-terminated alkanethiol monolayers from Au(111) surfaces. J. Electroanal. Chem. 1997, 428, 33–38. [Google Scholar]
- Hobara, D.; Miyake, K.; Imabayashi, S.; Niki, K.; Kakiuchi, T. In-Situ Scanning Tunneling Microscopy Imaging of the Reductive Desorption Process of Alkanethiols on Au(111). Langmuir 1998, 14, 3590–3596. [Google Scholar]
- Kakiuchi, T.; Usui, H.; Hobara, D.; Yamamoto, M. Voltammetric Properties of the Reductive Desorption of Alkanethiol Self-Assembled Monolayers from a Metal Surface. Langmuir 2002, 18, 5231–5238. [Google Scholar]
- Eggers, P.K.; Zareie, H.M.; Paddon-Row, M.N.; Gooding, J.J. Structure and Properties of Redox Active Self-Assembled Monolayers Formed from Norbornylogous Bridges. Langmuir 2009, 25, 11090–11096. [Google Scholar]
- A slightly higher energy (2.8 kJ•molࢤ1) of the syn conformer was also reported for parent 2,2′-bithiophene: Diaz-Quijada, G.A.; Weinberg, N.; Holdcroft, S.; Pinto, B.M. Investigation of Barriers To Conformational Interchange in Oligothiophenes and Oligo(Thienyl)furans. J. Phys. Chem. A 2002, 106, 1266–1276. [Google Scholar]
- Wakamiya, A.; Nishinaga, T.; Komatsu, K. The stable radical cation of thiophene annelated with bicyclo[2.2.2]octene and its reaction with triplet oxygen to give a protonated cation of 2-butene-1,4-dione derivative. Chem. Commun. 2002, 1192–1193. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2001; pp. 590–593. [Google Scholar]
- Dudek, S.P.; Sikes, H.D.; Chidsey, C.E.D. Synthesis of Ferrocenethiols Containing Oligo(phenylenevinylene) Bridges and Their Characterization on Gold Electrodes. J. Am. Chem. Soc. 2001, 123, 8033–8038. [Google Scholar]
- Lee, L.Y.S.; Sutherland, T.C.; Rucareanu, S.; Lennox, R.B. Ferrocenylalkylthiolates as a Probe of Heterogeneity in Binary Self-Assembled Monolayers on Gold. Langmuir 2006, 22, 4438–4444. [Google Scholar]
- Guo, Y.; Zhao, J.; Zhu, J. Study on the intermolecular interactions between the functional moieties inferrocene-terminated alkanethiol self-assembled monolayer on gold. Thin Solid Films 2008, 516, 3051–3057. [Google Scholar]
- The large current decrease in the second cycle may be ascribed to a double layer reorganization
- Van Haare, J.A.E.H.; van Boxtel, M.; Janssen, R.A.J. Dimers of Prototype High-Spin Polaronic Oligomers. Chem. Mater. 1998, 10, 1166–1175. [Google Scholar]
- Barner, B.J.; Green, M.J.; Saez, E.I.; Corn, R.M. Polarization modulation Fourier transform infrared reflectance measurements of thin films and monolayers at metal surfaces utilizing real-time sampling electronics. Anal. Chem. 1991, 63, 55–60. [Google Scholar]
- Yokooji, A.; Satoh, T.; Miura, M.; Nomura, M. Synthesis of 5,5′-diarylated 2,2′-bithiophenes viapalladium-catalyzed arylation reactions. Tetrahedron 2004, 60, 6757–6763. [Google Scholar]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004.
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kitagawa, T.; Matsubara, H.; Okazaki, T.; Komatsu, K. Electrochemistry of the Self-Assembled Monolayers of Dyads Consisting of Tripod-Shaped Trithiol and Bithiophene on Gold. Molecules 2014, 19, 15298-15313. https://doi.org/10.3390/molecules190915298
Kitagawa T, Matsubara H, Okazaki T, Komatsu K. Electrochemistry of the Self-Assembled Monolayers of Dyads Consisting of Tripod-Shaped Trithiol and Bithiophene on Gold. Molecules. 2014; 19(9):15298-15313. https://doi.org/10.3390/molecules190915298
Chicago/Turabian StyleKitagawa, Toshikazu, Hiroaki Matsubara, Takao Okazaki, and Koichi Komatsu. 2014. "Electrochemistry of the Self-Assembled Monolayers of Dyads Consisting of Tripod-Shaped Trithiol and Bithiophene on Gold" Molecules 19, no. 9: 15298-15313. https://doi.org/10.3390/molecules190915298