Electrospray Ionization Mass Spectrometric Analysis of Highly Reactive Glycosyl Halides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Preparation

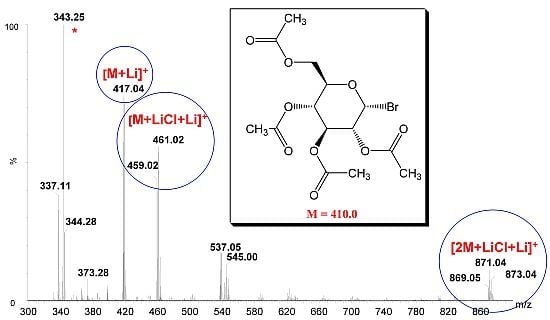
2.2. Measurement of Glycosyl Chloride and Bromide Samples


2.3. Accurate Mass Determination

| Analyte | Formula | Calculated m/z | Measured m/z | Mass difference (ppm) |
|---|---|---|---|---|
| 1 | C21H2135Cl7LiO5+ | 395.1238 | 395.1256 | 4.6 |
| 2 | C14H1979Br7LiO9+ | 417.0372 | 417.0386 | 3.4 |
3. Experimental
3.1. General
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Demchenko, A.V., Ed. Handbook of Chemical Glycosylation. Advances in Stereoselectivity and Therapeutic Relevance; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Fügedi, P. Glycosylation Methods. In The Organic Chemistry of Sugars; Levy, D.E., Fügedi, P., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 108–198. [Google Scholar]
- Nicolaou, K.C.; Ueno, H. Oligosaccharide Synthesis from Glycosyl Fluorides and Sulfides. In Preparative Carbohydrate Chemistry; Hanessian, S., Ed.; Marcel Dekker: New York, NY, USA, 1997; pp. 313–338. [Google Scholar]
- Yokoyama, M. Methods of synthesis of glycosyl fluorides. Carbohydr. Res. 2000, 327, 5–14. [Google Scholar] [CrossRef]
- Williams, S.J.; Withers, S.G. Glycosyl fluorides in enzymatic reactions. Carbohydr. Res. 2000, 327, 27–46. [Google Scholar] [CrossRef]
- Meloncelli, P.J.; Martin, A.D.; Lowary, T.L. Glycosyl iodides. History and recent advances. Carbohydr. Res. 2009, 344, 1110–1122. [Google Scholar] [CrossRef]
- Spencer, R.P.; Schwartz, J. Variously substituted glycals are readily prepared from glycosyl bromides using (Cp2TiCl)2. Tetrahedron Lett. 1996, 37, 4357–4360. [Google Scholar] [CrossRef]
- Ernst, B.; Winkler, T. Preparation of glycosyl halides under neutral conditions. Tetrahedron Lett. 1989, 30, 3081–3084. [Google Scholar] [CrossRef]
- Hung, S.C.; Wong, C.H. Synthesis of glycosyl chlorides with acid-labile protecting groups. Tetrahedron Lett. 1996, 37, 4903–4906. [Google Scholar]
- Kadentsev, V.I.; Chizhov, A.O.; Chizhov, O.S.; Voznyi, Y.V. Tandem electrospray mass spectrometry of acetates of glycosyl fluorides and deoxyfluorohexopyranoses (in Russian). Mass-Spektrom. 2008, 5. Available online: http://elibrary.ru/item.asp?id=9936469 (accessed on 16 May 2012).
- Kadentsev, V.I.; Chizhov, A.O.; Chizhov, O.S.; Voznyi, Y.V. Tandem electrospray mass spectrometry of benzoates of 2-substituted glycosyl fluorides (in Russian). Mass-Spektrom. 2008, 5, pp. 45–48. Available online: http://elibrary.ru/item.asp?id=9936470 (accessed on 16 May 2012).
- Kele, Z.; Kupihár, Z.; Kovács, L.; Janáky, T.; Szabó, P.T. Electrospray mass spectrometry of phosphoramidites, a group of acid-labile compounds. J. Mass Spectrom. 1999, 34, 1317–1321. [Google Scholar] [CrossRef]
- Kupihár, Z.; Timár, Z.; Darula, Z.; Dellinger, D.J.; Caruthers, M.H. An electrospray mass spectrometric method for accurate mass determination of highly acid-sensitive phosphoramidites. Rapid Commun. Mass Spectrom. 2008, 22, 533–540. [Google Scholar]
- Bhat, C.C. 2-Deoxy-3,5-di-O-p-toluoyl-D-erythro-pentosyl chloride. In Synthetic Procedures in Nucleic Acid Chemistry; Zorbach, W.W., Tipson, R.S., Eds.; John Wiley and Sons: New York, NY, USA, 1968; Volume 1, pp. 521–522. [Google Scholar]
- Osborn, H.M.I. Best Synthetic Methods: Carbohydrates; Academic Press: Amsterdam, The Netherlands, 2003; p. 78. [Google Scholar]
- Kronzer, F.J.; Schuerch, C. The use of 2,3,4,6-tetra-O-benzyl-alpha-d-glycopyranosyl iodides in alpha-glycoside synthesis. Carbohydr. Res. 1974, 34, 71–78. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Hendriks, K.B.; Stick, R.V.; James, K. Halide ion catalyzed glycosidation reactions. Syntheses of alpha-linked disaccharides. J. Am. Chem. Soc. 1975, 97, 4056–4062. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 5th ed; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Sample Availability: Samples of the compounds 1 and 2 are not stable enough for distribution.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bokros, A.; Bánfi, A.; Kupihár, Z.; Kele, Z.; Illyés, T.Z.; Szolomájer, J.; Kovács, L. Electrospray Ionization Mass Spectrometric Analysis of Highly Reactive Glycosyl Halides. Molecules 2012, 17, 8351-8358. https://doi.org/10.3390/molecules17078351
Bokros A, Bánfi A, Kupihár Z, Kele Z, Illyés TZ, Szolomájer J, Kovács L. Electrospray Ionization Mass Spectrometric Analysis of Highly Reactive Glycosyl Halides. Molecules. 2012; 17(7):8351-8358. https://doi.org/10.3390/molecules17078351
Chicago/Turabian StyleBokros, Attila, Annamária Bánfi, Zoltán Kupihár, Zoltán Kele, Tünde Zita Illyés, János Szolomájer, and Lajos Kovács. 2012. "Electrospray Ionization Mass Spectrometric Analysis of Highly Reactive Glycosyl Halides" Molecules 17, no. 7: 8351-8358. https://doi.org/10.3390/molecules17078351