The Role of Selected Lactic Acid Bacteria on Organic Acid Accumulation during Wet and Spray-Dried Fish-Based Silages. Contributions to the Winning Combination of Microbial Food Safety and Environmental Sustainability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstocks
2.2. Microbiological Enumeration, Isolation, and Identification of LAB Strains for the Silage Assays
2.2.1. Microbiological Enumeration
2.2.2. Microbiological Isolation of LAB Strains
2.2.3. Microbiological Culture Maintenance
2.2.4. Molecular Identification of LAB Strains
2.3. Preparation of Silages and Sampling
2.4. Extraction of Organic Acids
2.5. Separation, Identification, and Quantification of Organic Acids by RP-HPLC-DAD
2.5.1. RP-HPLC-DAD System and Elution Methodology
2.5.2. Preparation of Calibration Curves and Quantification of Organic Acids by RP-HPLC-DAD
2.6. Statistical Analysis
3. Results
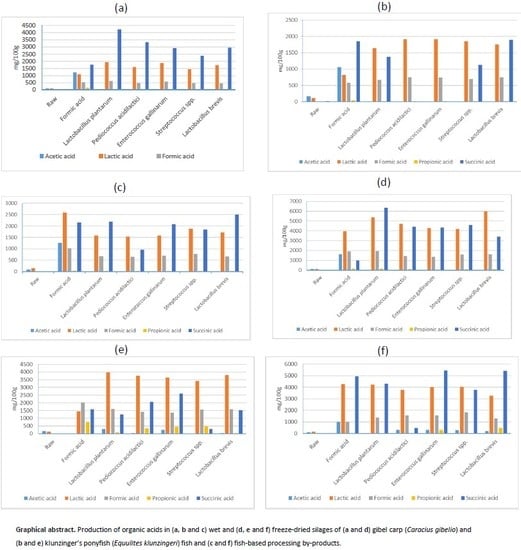
3.1. Organic Acids in Wet Fish-Based Silages
3.2. Organic Acids in Spray-Dried Fish-based Silages
4. Discussion
4.1. Organic Acids in Wet Fish-Based Silages
4.2. Organic Acids in Spray-Dried Fish-Based Silages
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2010. The World Review of Fisheries and Aquaculture; FAO: Rome, Italy, 2010. [Google Scholar]
- Tomczak-Wandzel, R.; Mędrzycka, K. Preparation, composition and properties of fish silage produced with post-coagulation sludge. Env. Prot. Eng. 2013, 39, 39–49. [Google Scholar]
- FAO. Animal Feed Resources Information System. 2014. Available online: http://www.fao.org (accessed on 11 March 2019).
- Hossain, U.; Alam, A. Production of powder fish silage from fish market wastes. SAARC J. Agr. 2016, 13, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.M.; Fairgrieve, W.T.; Skonberg, D.I.; Rasco, B.A. Preparation and nutrient analyses of lactic acid bacterial ensiled salmon viscera. Aquaculture 1993, 109, 351–366. [Google Scholar] [CrossRef]
- Fagbenro, O.; Jauncey, K.; Haylor, G. Nutritive value of diet containing dried lactic acid fermented fish silage and soybean meal for juvenile Oreochromis niloticus and Clarias gariepinus. Aquat. Living Resour. 1994, 7, 79–85. [Google Scholar] [CrossRef]
- Mousavi, S.L.; Mohammadi, G.; Khodadadi, M.; Keysami, M.A. Silage production from fish waste in cannery factories of Bushehr city using mineral acid, organic acid, and biological method. Int. J. Agr. Crop Sci. 2013, 6, 610–616. [Google Scholar]
- Green, S.; Wiseman, J.; Cole, D.J.A. Fish silage in pig diets. Pig News Inf. 1983, 4, 269–273. [Google Scholar]
- Machin, D.H.; Panigrahi, S.; Bainton, J.; Morris, T.R. Performance of broiler chicks fed on low and high oil fish silages in relation to changes taking place in lipid and protein components. Anim. Feed Sci. Technol. 1990, 28, 199–223. [Google Scholar] [CrossRef]
- Offer, N.W.; Husain, R.A.K. Fish silage as a protein supplement for early-weaned calves. Anim. Feed Sci. Tech. 1987, 17, 165–177. [Google Scholar] [CrossRef]
- Samuels, W.A.; Fontenot, J.P.; Allen, V.G.; Abazinge, M.D. Seafood processing wastes ensiled with straw: Utilization and intake by sheep. J. Anim. Sci. 1991, 69, 4983–4992. [Google Scholar] [CrossRef]
- Al-Abri, A.S.; Mahgoub, O.; Kadim, I.T.; Al-Marzooqi, W.; Goddard, S.J.; Al-Farsi, M. Processing and evaluation of nutritive value of fish silage for feeding Omani sheep. J. Appl. Anim. Res. 2014, 42, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Olstorpe, M.; Axelsson, L.; Schnurer, J.; Passoth, V. Effect of starter culture inoculation on feed hygiene and microbial population development in fermented pig feed composed of a cereal grain mix with wet wheat distillers’ grain. J. Appl. Microbiol. 2010, 108, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bannister, B.A.; Begg, N.T.; Gillespie, S.H. Infectious Disease, 2nd ed.; Blackwell Science: Malden, MA, USA, 2000. [Google Scholar]
- Schroeder, J.W. Silage Fermentation and Preservation; NDSU Extension Service: Fargo, ND, USA, 2004. [Google Scholar]
- Tarman, A.A.; Ramli, N.N.; Ridla, M.R.M.; Yaman, M.A.; Setiyono, A.A. Effects of organic acids on Salmonella enteritidis growth inhibition and ileum surface area in laying ducks fed anaerobically fermented feed. Int. J. Poultry Sci. 2017, 16, 98–104. [Google Scholar]
- Luckstadt, C.; Mellor, S. Holoanalysis—The acid test in pig diets. Kraftfutter Feed Mag. 2010, 1, 18–21. [Google Scholar]
- Suiryanrayna, M.V.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.R.; Hall, C.J. Growth inhibition of food-borne pathogens by lactic and acetic acids and their mixtures. Int. J. Food Sci. Technol. 1988, 23, 287–292. [Google Scholar] [CrossRef]
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Oliveira, J.S.D.; Queiroz, A.C.D.; Mantovani, H.C.; Melo, M.R.D.; Detmann, E.; Santos, E.M.; Bayão, G.F.V. Effect of propionic and lactic acids on in vitro ruminal bacteria growth. R. Bras. Zootec. 2011, 40, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Moon, G.S.; Kim, W.J.; Kim, M. Synergistic effects of bacteriocin-producing Pediococcus acidilactici K10 and organic acids on inhibiting Escherichia coli O157: H7 and applications in ground beef. J. Microbiol. Biotechnol. 2002, 12, 936–942. [Google Scholar]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.P.; Begin, A. Inhibitory effect of organic acids upon meat spoilage bacteria. J. Food Prot. 1997, 60, 246–253. [Google Scholar] [CrossRef]
- Knarreborg, A.; Miquel, N.; Granli, T.; Jensen, B.B. In vitro methodology to evaluate the effect of various additives on the microflora in gastrointestinal tract of pigs. In Digestive Physiology in Pigs: Proceedings of the 8th Symposium; Lindberg, J.E., Ogle, B., Eds.; CABI Publishing: Oxon, UK, 2001; pp. 302–304. [Google Scholar]
- Naughton, P.J.; Jensen, B.B. A bioreactor system to study survival of Salmonella Typhimurium in pig gut content. Berl. Münch. Tierärztl 2001, 114, 1–4. [Google Scholar]
- Jensen, B.B.; Mikkelsen, L.L.; Canibe, N.; Høyberg, O. Annual Report 2001: Salmonella in Slaughter Pigs; Danish Institute of Agricultural Sciences, Research Centre Foulum: Tjele, Denmark, 2001; p. 23. [Google Scholar]
- Rudbäck, L. Organic Acids in Liquid Feed for Pigs-Palatability and Feed Intake. MSc Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2013. [Google Scholar]
- Alp, M.; Kocabagli, N.; Kahraman, R.; Bostan, K. Effects of dietary supplementation with organic acids and zinc bacitracin on ileal microflora, pH and performance in broilers. Turk. J. Vet. Anim. Sci. 1999, 23, 451–455. [Google Scholar]
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluge, H.; Broz, J.; Eder, K. Effect of benzoic acid on growth performance, nutrient digestibility, nitrogen balance, gastrointestinal microflora and parameters of microbial metabolism in piglets. J. Anim. Physiol. Anim. Nutr. 2006, 90, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.K.; Koh, C.B. The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev. Aquac. 2017, 9, 342–368. [Google Scholar] [CrossRef]
- Ozyurt, G. Biotransformation of Fisheries Byproducts Fermented with Natural Lactic Acid Bacteria, the Quality of Fermentation Products and Their Use in Animal Feeding; Final Report of TUBITAK Project (213O166); TUBITAK: Ankara, Turkey, 2016; p. 128. [Google Scholar]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Nat. Ac. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef] [Green Version]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried Marion berry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- De-Baere, S.; Eeckhaut, V.; Steppe, M.; De Maesschalck, C.; De Backer, P.; Van Immerseel, F.; Croubels, S. Development of a HPLC-UV method for the quantitative determination of four short-chain fatty acids and lactic acid produced by intestinal bacteria during in vitro fermentation. J. Pharm. Biomed. Anal. 2013, 80, 107–115. [Google Scholar] [CrossRef]
- Özcelik, S.; Kuley, E.; Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT-Food Sci. Technol. 2016, 73, 536–542. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Nogueira, M.; Duran, A.; Priet, M.A.; Rodriguez-Amado, I.; Rial, D.; Murado, M.A. Preparation of marine silage of swordfish, ray and shark visceral waste by lactic acid bacteria. J. Food Eng. 2011, 103, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Wildan, F. Determination of lactic acid levels in the silage and liquid rumen by gas chromatography. In Proceedings of the National Technical Meeting of Agricultural Functional Staff, Jawa Barat, Indonesia, 3 August 2004; Centre for Research and Development of Animal Husbandry; Jawa Barat, Indonesia, 2005; pp. 100–103. [Google Scholar]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Shirai, K.; Guerrero, I.; Huerta, S.; Saucedo, G.; Castillo, A.; Gonzalez, R.O.; Hall, G.M. Effect of initial glucose concentration and inoculation level of lactic acid bacteria in shrimp waste ensilation. Enzyme Microb. Tech. 2001, 28, 446–452. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Dong, Z.; Li, J.; Guo, G.; Bai, Y.; Shao, T. Characteristics of isolated lactic acid bacteria and their effects on the silage quality. Asian-Australas. J. Anim. Sci. 2017, 30, 819–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driehuis, F.; Elferink, S.; Spoelstra, S. Anaerobic lactic acid degradation during ensilage of whole crop maize inoculated with Lactobacillus buchneri inhibits yeast growth and improves aerobic stability. J. Appl. Microbiol. 1999, 87, 583–594. [Google Scholar] [CrossRef]
- Ahmadi, N.; Khosravi-Darani, K.; Zarean-Shahraki, S.; Mortazavian, M.; Mashayekh, S.M. Fed-batch fermentation for propionic, acetic and lactic acid production. Orient. J. Chem. 2015, 31, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Abdul Rahman, N.; Halim, A.; Ridzwan, M.; Mahawi, N.; Hasnudin, H.; Al-Obaidi, J.R.; Abdullah, N. Determination of the use of Lactobacillus plantarum and Propionibacteriumfreudenreichii application on fermentation profile and chemical composition of corn silage. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rowghani, E.; Zamiri, M.J.; Khorvash, M.; Abdollahipanah, A. The effects of Lactobacillus plantarum and Propionibacterium acidipropionici on corn silage fermentation, ruminal degradability and nutrient digestibility in sheep. Iran. J. Vet. Res. 2008, 9, 308–315. [Google Scholar]
- Chen, L.; Guo, G.; Yuan, X.; Zhang, J.; Li, J.; Shao, T. Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat–common vetch intercrop on the Tibetan Plateau. J. Sci. Food Agric. 2016, 96, 1678–1685. [Google Scholar] [CrossRef]
- Wagner, N.; Tran, Q.H.; Richter, H.; Selzer, P.M.; Unden, G. Pyruvate fermentation by Oenococcus oeni and Leuconostoc mesenteroides and role of pyruvate dehydrogenase in anaerobic fermentation. Appl. Env. Microbiol. 2005, 71, 4966–4971. [Google Scholar] [CrossRef] [Green Version]
- Goffin, P.; Muscariell, L.; Lorquet, F.; Stukkens, A.; Prozzi, D.; Sacco, M.; Kleerebezem, M.; Hols, P. Involvement of pyruvate oxidase activity and acetate production in the survival of Lactobacillus plantarum during the stationary phase of aerobic growth. Appl. Env. Microbiol. 2006, 72, 7933–7940. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.M. Rumen Microbiology and Its Role in Ruminant Nutrition; Ithaca: New York, NY, USA, 2002. [Google Scholar]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Mayrhofer, S.; Domig, K.; Starkute1, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; et al. Inhibition of pathogenic and opportunistic bacteria and molds by lactic acid bacteria isolated from spontaneous baking sourdough. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.C.; Lee, S.Y.; Hong, S.H.; Chang, H.N. Isolation and characterization of new succinic acid producing bacterium, Mannheimia succiniciproducens MBEL 55E, from bovine rumen. Appl. Microbiol. Biotechnol. 2002, 58, 663–668. [Google Scholar] [PubMed]
- Lin, H.; Bennett, G.N.; San, K.Y. Fed-batch culture of a metabolically engineered Escherichia coli strain designed for high-level succinate production and yield under aerobic conditions. Biotechnol. Bioeng. 2005, 90, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Bretz, K.; Kabasci, S. Feed-control development for succinic acid production with Anaerobiospirillumsucciniciproducens. Biotechnol. Bioeng. 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Skrivanova, E.; Marounek, M.; Benda, V.; Brezina, P. Susceptibility of Escherichia coli, Salmonella sp and Clostridium perfringens to organic acids and monolaurin. Vet. Med. 2006, 51, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.N.; Chowdhury, R.; Islam, K.M.S.; Akbar, M.A. Propionic acid is an alternative to antibiotics in poultry diet. Bangl. J. Anim. Sci. 2009, 38, 115–122. [Google Scholar] [CrossRef]

| Type of Raw-Material | Type of Preprocessing of Raw-Material | Type of Silage | Organic Acids |
|---|---|---|---|
| - Gibel carp - Klunzinger’s ponyfish - Fish processing by-products | - Wet - Spray-dried | - Acidified fish silage with 3% formic acid - Fermented silage with Enterococcus gallinarum - Fermented silage with Lactobacillus brevis - Fermented silage with Lactobacillus plantarum - Fermented silage with Pediococcus acidilactici - Fermented silage with Streptococcus spp. | - Acetic acid - Formic acid - Lactic acid - Propionic acid - Succinic acid |
| Time (min) | %A | %B | Flow-Rate (mL/min) |
|---|---|---|---|
| 0 | 0 | 100 | 0.8 |
| 6 | 0 | 100 | 1 |
| 9 | 35 | 65 | 0.8 |
| 10 | 2.5 | 97.5 | 0.8 |
| 15 | 0 | 100 | 0.8 |
| 20 | 0 | 100 | 0.8 |
| Organic Acid | Calibration Curve | Linear Correlation Coefficient | n | Retention Time (RT) (min) | %RSD |
|---|---|---|---|---|---|
| Acetic acid [CH3COOH] | Y = (2.71176 × 10−6) X | 0.99997 | 9 | 4.77 | 5.414 |
| Formic acid [HCOOH] | Y = (2.01452 × 10−6) X | 0.99999 | 9 | 3.17 | 7.206 |
| Lactic acid [CH3CH(OH)COOH] | Y = (3.57439 × 10−6) X | 0.99995 | 9 | 4.50 | 6.670 |
| Propionic acid [CH3CH2COOH] | Y = (4.33284 × 10−6) X | 0.99999 | 9 | 10.17 | 0.996 |
| Succinic acid [(CH2)2(COOH)2] | Y = (2.52321 × 10−6) X | 0.99999 | 9 | 7.57 | 10.169 |
| Processing Conditions | Acetic Acid | Formic Acid | Lactic Acid | Propionic Acid | Succinic Acid |
|---|---|---|---|---|---|
| Raw-material | 104.30 ± 9.14 | 0.00 ± 0.00 | 109.88 ± 4.14 | 0.00 ± 0.00 | 10.09 ± 0.56 |
| Formic acid 3% (v/w) | 528.76 ± 26.19 | 1227.38 ± 17.38 * | 1098.22 ± 13.67 | 1762.34 ± 17.45 | 152.87 ± 0.21 |
| Enterococcus gallinarum | 577.73 ± 3.61 c | 0.00 ± 0.00 | 1876.38 ± 13.41 d | 2916.82 ± 31.16 b | 17.99 ± 0.50 c |
| Lactobacillus brevis | 447.15 ± 26.24 a | 0.00 ± 0.00 | 1730.57 ± 19.91 c | 2947.25 ± 31.95 b | 15.56 ± 0.67 b |
| Lactobacillus plantarum | 621.29 ± 2.79 d | 0.00 ± 0.00 | 1935.43 ± 15.98 e | 4218.80 ± 5.96 d | 19.53 ± 1.02 d |
| Pediococcus acidilactici | 471.32 ± 8.75 ab | 0.00 ± 0.00 | 1596.90 ± 6.47 b | 3325.93 ± 8.26 c | 16.82 ± 0.83 bc |
| Streptococcus spp. | 476.75 ± 17.27 b | 0.00 ± 0.00 | 1442.15 ± 8.67 a | 2375.95 ± 11.06 a | 12.95 ± 1.02 a |
| Processing Conditions | Acetic Acid | Formic Acid | Lactic Acid | Propionic Acid | Succinic Acid |
|---|---|---|---|---|---|
| Raw-material | 173.87 ± 11.65 | 0.00 ± 0.00 | 113.41 ± 5.14 | 0.00 ± 0.00 | 14.52 ± 0.24 |
| Formic acid 3% (v/w) | 584.49 ± 0.88 | 1051.13 ± 5.16 * | 810.23 ± 20.90 | 1851.06 ± 14.87 | 43.62 ± 0.65 |
| Enterococcus gallinarum | 735.88 ± 7.20 b | 3.25 ± 0.16 b | 1909.39 ± 26.47 d | 0.00 ± 0.00 | 13.29 ± 0.59 a |
| Lactobacillus brevis | 697.11 ± 4.23 a | 2.76 ± 0.02 a | 1848.21 ± 7.05 c | 1133.52 ± 19.00 a | 12.92 ± 0.66 a |
| Lactobacillus plantarum | 674.19 ± 26.09 a | 0.00 ± 0.00 | 1637.14 ± 1.86 a | 1372.62 ± 8.24 b | 13.78 ± 0.99 a |
| Pediococcus acidilactici | 743.23 ± 10.60 b | 0.00 ± 0.00 | 1913.39 ± 30.46 d | 0.00 ± 0.00 | 13.33 ± 0.54 a |
| Streptococcus spp. | 752.11 ± 9.33 b | 2.90 ± 0.11 a | 1751.93 ± 10.97 b | 1894.01 ± 17.29 c | 12.94 ± 0.74 a |
| Processing Conditions | Acetic Acid | Formic Acid | Lactic Acid | Propionic Acid | Succinic Acid |
|---|---|---|---|---|---|
| Raw-material | 87.23 ± 3.54 | 0.00 ± 0.00 | 138.68 ± 2.83 | 0.00 ± 0.00 | 11.56 ± 1.03 |
| Formic acid 3% (v/w) | 1022.62 ± 55.55 | 1253.89 ± 22.0 * | 2588.74 ± 23.05 | 2149.37 ± 17.89 | 35.99 ± 1.66 |
| Enterococcus gallinarum | 703.10 ± 18.02 b | 2.62 ± 0.15 a | 1587.69 ± 11.97 a | 2080.19 ± 47.39 c | 20.79 ± 1.37 a |
| Lactobacillus brevis | 770.64 ± 15.38 c | 4.03 ± 0.35 c | 1885.46 ± 83.41 c | 1842.22 ± 25.63 b | 26.52 ± 0.87 c |
| Lactobacillus plantarum | 674.20 ± 27.19 ab | 0.00 ± 0.00 | 1581.40 ± 21.68 a | 2191.49 ± 34.61 d | 23.42 ± 1.42 b |
| Pediococcus acidilactici | 646.94 ± 17.11 a | 3.29 ± 0.19 b | 1526.80 ± 38.71 a | 960.73 ± 22.48 a | 21.22 ± 0.36 a |
| Streptococcus spp. | 655.31 ± 3.75 a | 2.30 ± 0.16 a | 1719.45 ± 9.96 b | 2505.18 ± 17.95 e | 22.38 ± 0.25 ab |
| Processing Conditions | Acetic Acid | Formic Acid | Lactic Acid | Propionic Acid | Succinic Acid |
|---|---|---|---|---|---|
| Formic acid 3% (v/w) | 1917.20 ± 50.12 | 1611.37 ± 119.2 * | 3958.32 ± 50.35 | 987.44 ± 84.79 | 141.74 ± 5.33 |
| Enterococcus gallinarum | 1361.89 ± 54.27 a | 0.00 ± 0.00 | 4259.89 ± 77.10 a | 4311.11 ± 154.52 b | 55.53 ± 0.81 a |
| Lactobacillus brevis | 1606.32 ± 39.93 b | 8.76 ± 0.46 | 6003.48 ± 131.11 d | 3397.95 ± 160.78 a | 82.41 ± 3.71 b |
| Lactobacillus plantarum | 1939.35 ± 116.34 c | 0.00 ± 0.00 | 5363.48 ± 83.14 c | 6335.40 ± 157.98 d | 134.13 ± 9.27 d |
| Pediococcus acidilactici | 1417.19 ± 65.34 a | 0.00 ± 0.00 | 4705.52 ± 196.59 b | 4421.03 ± 116.73 bc | 96.95 ± 8.11 c |
| Streptococcus spp. | 1580.17 ± 26.41 b | 0.00 ± 0.00 | 4192.19 ± 50.53 a | 4569.54 ± 63.80 c | 75.12 ± 4.58 b |
| Processing Conditions | Acetic Acid | Formic Acid | Lactic Acid | Propionic Acid | Succinic Acid |
|---|---|---|---|---|---|
| Formic acid 3% (v/w) | 2030.62 ± 31.62 | 1212.54 ± 10.8 * | 1426.59 ± 13.99 | 1595.92 ± 30.25 | 750.88 ± 46.49 |
| Enterococcus gallinarum | 1359.12 ± 36.85 a | 253.99 ± 13.91 b | 3647.40 ± 79.28 b | 2605.53 ± 138.81 e | 454.29 ± 16.11 d |
| Lactobacillus brevis | 1564.00 ± 24.81 b | 18.59 ± 0.81 a | 3431.80 ± 99.60 a | 411.78 ± 0.55 a | 479.69 ± 21.48 e |
| Lactobacillus plantarum | 1602.10 ± 19.60 b | 301.32 ± 2.57 c | 3975.00 ± 64.29 c | 1247.41 ± 27.50 b | 66.85 ± 3.16 b |
| Pediococcus acidilactici | 1408.05 ± 42.66 a | 26.51 ± 1.98 a | 3755.22 ± 143.65 b | 2063.86 ± 89.28 d | 346.46 ± 10.48 c |
| Streptococcus spp. | 1579.27 ± 114.73 b | 27.71 ± 0.70 a | 3805.97 ± 71.29 bc | 1530.73 ± 27.41 c | 38.80 ± 2.11 a |
| Processing Conditions | Lactic Acid | Formic Acid | Acetic Acid | Propionic Acid | Succinic Acid |
|---|---|---|---|---|---|
| Formic acid 3% (v/w) | 4259.58 ± 61.93 | 1005.90x ± 22.75y * | 1006.82 ± 20.38 | 4928.54 ± 88.20 | 37.38 ± 1.61 |
| Enterococcus gallinarum | 3987.31 ± 77.38 c | 321.44 ± 10.49 d | 1574.57 ± 14.14 c | 5464.99 ± 96.47 d | 308.51 ± 3.93 c |
| Lactobacillus brevis | 4036.68 ± 99.09 c | 290.46 ± 7.26 c | 1833.95 ± 20.18 d | 3779.11 ± 41.57 b | 25.93 ± 0.83 b |
| Lactobacillus plantarum | 4218.92 ± 24.03 d | 21.98 ± 0.87 a | 1370.63 ± 35.40 b | 4292.87 ± 89.12 c | 18.15 ± 0.94 ab |
| Pediococcus acidilactici | 3780.18 ± 16.50 b | 317.61 ± 14.80 d | 1587.63 ± 5.09 c | 474.66 ± 24.33 a | 12.80 ± 1.41 a |
| Streptococcus spp. | 3259.16 ± 45.33 a | 217.81 ± 4.02 b | 1289.77 ± 17.11 a | 5421.72 ± 19.83 d | 465.77 ± 12.97 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuley, E.; Özyurt, G.; Özogul, I.; Boga, M.; Akyol, I.; Rocha, J.M.; Özogul, F. The Role of Selected Lactic Acid Bacteria on Organic Acid Accumulation during Wet and Spray-Dried Fish-Based Silages. Contributions to the Winning Combination of Microbial Food Safety and Environmental Sustainability. Microorganisms 2020, 8, 172. https://doi.org/10.3390/microorganisms8020172
Kuley E, Özyurt G, Özogul I, Boga M, Akyol I, Rocha JM, Özogul F. The Role of Selected Lactic Acid Bacteria on Organic Acid Accumulation during Wet and Spray-Dried Fish-Based Silages. Contributions to the Winning Combination of Microbial Food Safety and Environmental Sustainability. Microorganisms. 2020; 8(2):172. https://doi.org/10.3390/microorganisms8020172
Chicago/Turabian StyleKuley, Esmeray, Gulsun Özyurt, Ilyas Özogul, Mustafa Boga, Ismail Akyol, João M. Rocha, and Fatih Özogul. 2020. "The Role of Selected Lactic Acid Bacteria on Organic Acid Accumulation during Wet and Spray-Dried Fish-Based Silages. Contributions to the Winning Combination of Microbial Food Safety and Environmental Sustainability" Microorganisms 8, no. 2: 172. https://doi.org/10.3390/microorganisms8020172