Untargeted Metabolomics Revealed Potential Biomarkers of Small Yellow Follicles of Chickens during Sexual Maturation
Abstract
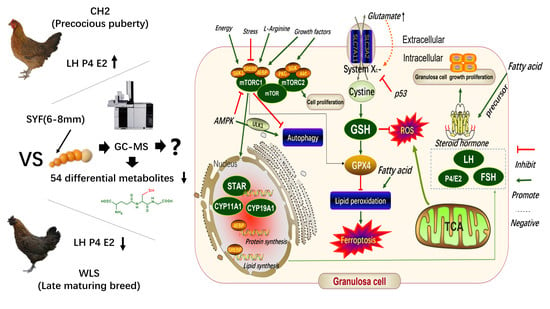
:1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Animals and Experimental Design
2.3. Sample Collection and Preparation
2.4. Liquid Chromatography–Mass Spectrometry (LC-MS)-Based Differential Metabolites and Metabolic Pathway Analysis
2.5. RNA Extraction and Quantitative Real-Time PCR (qPCR) Analysis
2.6. Histology Examination and Immunohistochemistry Analysis
2.7. Statistical Analysis
3. Results
3.1. The Determination of LH, P4, and E2 Secretion Levels in Serum and Small Yellow Follicle Tissue Using Enzyme-Linked Immunosorbent Assay (ELISA)
3.2. Expression Levels of Genes Associated with Sexual Maturation in Chicken SYF Tissue
3.3. Identification of Potential Differential Metabolites in SYF Tissue
3.4. Differential Metabolic Pathway Analysis in SYF Tissue
3.5. Expression Levels of Genes and Proteins Associated with Oxidative Stress in Chicken SYF Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, H.; Li, D.; Tian, Y.; Wei, Q.; Amevor, F.K.; Sun, C.; Yu, C.; Yang, C.; Du, H.; Jiang, X.; et al. miRNA sequencing analysis of healthy and atretic follicles of chickens revealed that miR-30a-5p inhibits granulosa cell death via targeting Beclin1. J. Anim. Sci. Biotechnol. 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Onagbesan, O.; Bruggeman, V.; Decuypere, E. Intra-ovarian Growth Factors Regulating Ovarian Function in Avian Species: A Review. J. Anim. Reprod. Sci. 2009, 111, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Robinson, F.E.; Renema, R.A.; Oosterhoff, H.H.; Zuidhof, M.J.; Wilson, J.L. Carcass Traits, Ovarian Morphology and Egg Laying Characteristics in Early Versus Late Maturing Strains of Commercial Egg-Type Hens. Poult. Sci. 2001, 80, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Li, Q.; Li, G.; Li, W.; Li, H.; Kang, X.; Tian, Y. Novel Regulatory Factors in the Hypothalamic-Pituitary-Ovarian Axis of Hens at Four Developmental Stages. Front Genet. 2020, 11, 591672. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Liu, Z.; Guo, X.; Du, Y.; Yuan, Z.; Guo, M.; Kang, L.; Sun, Y.; Jiang, Y. Transcriptome Analysis on Single Small Yellow Follicles Reveals That Wnt4 Is Involved in Chicken Follicle Selection. Front Endocrinol. 2017, 8, 317. [Google Scholar] [CrossRef] [Green Version]
- Lin, X. Degradation and Function of the Regressed Ovarian Follicle in the laying Chickens. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2018. [Google Scholar]
- Sechman, A.; Grzegorzewska, A.K.; Grzesiak, M.; Kozubek, A.; Katarzyńska-Banasik, D.; Kowalik, K.; Hrabia, A. Nitrophenols suppress steroidogenesis in pre-hierarchical chicken ovarian follicles by targeting STAR, HSD3B1, and CYP19A1 and downregulating LH and estrogen receptor expression. Domest Anim. Endocrinol. 2020, 70, 106378. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, G.; Lin, X.; Zhao, D.; Zhang, C.; Mi, Y. Reciprocal stimulating effects of bFGF and FSH on chicken primordial follicle activation through AKT and ERK pathway. Theriogenology 2019, 132, 27–35. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Chimbaka, I.M.; Qin, N.; Xu, X.; Liswaniso, S.; Xu, R.; Gonzalez, J.M. Transcriptome Analysis of Ovarian Follicles Reveals Potential Pivotal Genes Associated with Increased and Decreased Rates of Chicken Egg Production. Front Genet. 2021, 12, 622751. [Google Scholar] [CrossRef]
- Kim, D.; Johnson, A.L. Differentiation of the granulosa layer from hen pre-hierarchal follicles associated with follicle-stimulating hormone receptor signaling. Mol. Reprod. Dev. 2018, 85, 729–737. [Google Scholar] [CrossRef]
- Nitta, H.; Osawa, Y.; Bahr, J.M. Multiple steroidogenic cell populations in the thecal layer of preovulatory follicles of the chicken ovary. Endocrinology 1991, 129, 2033–2040. [Google Scholar] [CrossRef]
- Cui, Z.; Amevor, F.K.; Feng, Q.; Kang, X.; Song, W.; Zhu, Q.; Wang, Y.; Li, D.Y.; Zhao, X.L. Sexual Maturity Promotes Yolk Precursor Synthesis and Follicle Development in Hens via Liver-Blood-Ovary Signal Axis. Animals 2020, 10, 2348. [Google Scholar] [CrossRef] [PubMed]
- Hrabia, A.; Sechman, A.; Gertler, A.; Rząsa, J. Effect of Growth Hormone on Steroid Content, Proliferation and Apoptosis in the Chicken Ovary during Sexual Maturation. J. Cell Tissue Res. 2011, 345, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Ovarian follicle selection and granulosa cell differentiation. Poult Sci. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Zhang, Y.; Zhang, N.; Zang, L.; Wang, M.; Cui, X.; Jiang, Y. Identification of differentially expressed genes in ovaries of chicken attaining sexual maturity at different ages. Mol. Biol. Rep. 2012, 39, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, W.; Huang, T.; Gong, Y. Growth differentiation factor 9 promotes follicle-stimulating hormone-induced progesterone production in chicken follicular granulosa cells. Gen. Comp. Endocrinol. 2019, 276, 69–76. [Google Scholar] [CrossRef]
- Tang, S.; Li, X.; Wu, X.; Gong, Y. WT1 suppresses follicle-stimulating hormone-induced progesterone secretion by regulating ERK1/2 pathway in chicken preovulatory granulosa cells. Genes 2022, 812, 146097. [Google Scholar] [CrossRef]
- Qin, N.; Tyasi, T.L.; Sun, X.; Chen, X.; Zhu, H.; Zhao, J.; Xu, R. Determination of the roles of GREM1 gene in granulosa cell proliferation and steroidogenesis of hen ovarian prehierarchical follicles. Theriogenology 2020, 151, 28–40. [Google Scholar] [CrossRef]
- Du, H.; Guo, Y.; Wu, X.; Gong, Y. FOXL2 regulates the expression of the COL4A1 collagen gene in chicken granulosa cells. Mol. Reprod. Dev. 2022, 89, 95–103. [Google Scholar] [CrossRef]
- Ahumada-Solórzano, S.M.; Martínez-Moreno, C.G.; Carranza, M.; Ávila-Mendoza, J.; Luna-Acosta, J.L.; Harvey, S.; Luna, M.; Arámburo, C. Autocrine/paracrine proliferative effect of ovarian GH and IGF-I in chicken granulosa cell cultures. Gen. Comp. Endocrinol. 2016, 234, 47–56. [Google Scholar] [CrossRef]
- Lin, J.X.; Jia, Y.D.; Zhang, C.Q. Effect of epidermal growth factor on follicle-stimulating hormone-induced proliferation of granulosa cells from chicken pre-hierarchical follicles. J. Zhejiang Univ. Sci. 2011, 11, 875–883. [Google Scholar] [CrossRef]
- Kim, D.; Johnson, A.L. Vasoactive intestinal peptide promotes differentiation and clock gene expression in granulosa cells from pre-hierarchal follicles. Mol. Reprod. Dev. 2016, 85, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Li, J.; He, H.; Cao, Y.; Li, D.; Amevor, F.K.; Zhang, Y.; Wang, J.; Yu, C.; Yang, C.; et al. miR-23b-3p inhibits chicken granulosa cell proliferation and steroid hormone synthesis via targeting GDF9. Theriogenology 2022, 177, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, N.; Li, J.; Zhang, Z.; Guo, Y.; Li, D.; Zhang, Y.; Gong, Y.; Jiang, R.; Li, H.; et al. gga-miR-449b-5p Regulates Steroid Hormone Synthesis in Laying Hen Ovarian Granulosa Cells by Targeting the IGF2BP3 Gene. Animals 2022, 12, 2710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J.; Lei, Q.; Han, H.; Liu, W.; Cun, W.T.; Li, F.; Cao, D. Transcriptome Analysis of the Chicken Follicular Theca Cells with miR-135a-5p Suppressed. G3 Genes Genomes Genet. 2020, 10, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkersand towards mechanisms. J. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrum. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolmics-the link between genotypes and phenotypes. Plant Mol. Bio. 2022, 48, 155–171. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A. Development of a Robust and Repeatable UPLC-MS Method for the Long-Term Metabolomic Study of Human Serum. J. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Wishart, D.S.; Dan, T.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Fung, C.; Block, D.; Lewis, M.; Guo, N.; et al. HMDB: The human metabolome database. J. Nucleic Acids Res. 2007, 35, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K. MassBank: A public repository for sharing mass spectral data for life sciences. Int. J. Mass Spectr. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W. LMSD: LIPID MAPS structure database. J. Nucleic Acids Res. 2007, 35, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelrazig, S.; Safo, L.; Rance, G.A.; Fay, M.W.; Theodosiou, E.; Topham, P.D.; Kim, D.H.; Fernández-Castané, A. Metabolic characterisation of Magnetospirillum gryphiswaldense MSR-1 using LC-MS-based metabolite profiling. J. RSC Adv. 2020, 10, 32548–32560. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. J. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways frommetabolomic data using MetaboAnalyst. Nat. Protocol. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Miao, Z.; Teng, X.; Xu, S. Melatonin alleviates lead-induced intestinal epithelial cell pyroptosis in the common carps (Cyprinus carpio) via miR-17-5p/TXNIP axis. Fish Shellfish. Immunol. 2022, 131, 127–136. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.; Teng, X.; Luan, P.; Teng, X.; Yin, X. Immunosuppression participated in complement activation-mediated inflammatory injury caused by 4-octylphenol via TLR7/IκBα/NF-κB pathway in common carp (Cyprinus carpio) gills. Aquat. Toxicol. 2022, 249, 106211. [Google Scholar] [CrossRef]
- Mabuchi, R.; Adachi, M.; Ishimaru, A.; Zhao, H.; Tanimoto, S. Changes in Metabolic Profiles of Yellow tail (Seriola Quinqueradiata) Muscle during Cold Storage as a Freshness Evaluation Tool Based on GC-MS Metabolomics. Foods 2019, 8, 511. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Sun, Y.; Li, Y.; Wang, P.; Shi, L.; Ni, A.; Zong, Y.; Zhao, J.; Bian, S.; et al. Genetic Basis of Sexual Maturation Heterosis: Insights From Ovary lncRNA and mRNA Repertoire in Chicken. Front Endocrinol. 2022, 13, 951534. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.J. Photoperiodic Control of Reproduction in the Domestic Hen1. Poult. Sci. 1993, 72, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliff, E.A.; Dahl, R.E.; Pollak, S.D. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 2009, 80, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.L.; Solovieva, E.V.; Bridgham, J.T. Relationship between steroidogenic acute regulatory protein expression and progesterone production in hen granulosa cells during follicle development. Biol. Reprod. 2002, 67, 1313. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Ma, Y.; Zhao, D.; Mi, Y.; Zhang, C. Transcriptome profiling analysis of underlying regulation of growing follicle development in the chicken. Poult Sci. 2020, 99, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, L.; Han, K.; Zhang, X.; Zhang, G.; Dai, G.; Wang, J.; Xie, K. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. Anim. Reprod. Sci. 2019, 208, 106114. [Google Scholar] [CrossRef] [PubMed]
- Lew, P.D.; Tyler, N.C.; Gous, R.M.; Dunn, I.C.; Sharp, P.J. Photoperiodic response curves for plasma LH concentrations and age at first egg in female broiler breeders. Anim. Reprod. Sci. 2008, 109, 274–286. [Google Scholar]
- Dunn, I.C.; Lewis, P.D.; Wilson, P.W.; Sharp, P.J. Acceleration of maturation of FSH and LH responses to photostimulation in prepubertal domestic domestic hens by–oestrogen. Reproduction 2003, 126, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Ning, Z.; Deng, X.; Du, X.; Amevor, F.K.; Liu, L.; Kang, X.; Tian, Y.; Wang, Y.; Li, D.; et al. Integrated Proteomic and Metabolomic Analyses of Chicken Ovary Revealed the Crucial Role of Lipoprotein Lipase on Lipid Metabolism and Steroidogenesis During Sexual Maturity. Front. Physiol. 2022, 13, 885030. [Google Scholar]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell. Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef]
- Yuan, C.; Bu, X.C.; Yan, H.X.; Lu, J.J.; Zou, X.T. Dietary L-arginine levels affect the liver protein turnover and alter the expression of genes related to protein synthesis and proteolysis of laying hens. Poult. Sci. 2016, 95, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ragy, M.M.; Abdel-Hamid, H.A.; Toni, N.D.M. Pathophysiological changes in experimental polycystic ovary syndrome in female albino rats: Using either hemin or L-arginine. J. Cell Physiol. 2019, 234, 8426–8435. [Google Scholar] [CrossRef] [PubMed]
- Banh, R.S.; Kim, E.S.; Spillier, Q.; Biancur, D.E.; Yamamoto, K.; Sohn, A.S.W.; Shi, G.; Jones, D.; Kimmelman, A.C.; Pacold, M.E. The polar oxy-metabolome reveals the 4-hydroxymandelate CoQ10 synthesis pathway. Nature 2021, 597, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020, 21, 6695. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Jiang, R.; Xue, R.; Yin, X.; Wu, M.; Meng, Q. Ferroptosis in liver disease: New insights into disease mechanisms. Cell Death Discov. 2021, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. J. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Nie, R.; Zheng, X.; Zhang, W.; Zhang, B.; Ling, Y.; Zhang, H.; Wu, C. Morphological Characteristics and Transcriptome Landscapes of Chicken Follicles during Selective Development. Animals 2022, 12, 713. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, Y.; Liu, C.; Jiang, Y.; Kang, L. A Novel Single-Nucleotide Polymorphism in WNT4 Promoter Affects Its Transcription and Response to FSH in Chicken Follicles. Genes 2022, 13, 1774. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Li, J.; Feng, Y.; Gong, Y. Transcriptome analysis of the potential roles of FOXL2 in chicken pre-hierarchical and pre-ovulatory granulosa cells. Genomics 2017, 21, 56–66. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Q.; Yu, M.; Liu, Y.; Teng, X.H.; Gu, X.H. 4-tert-butylphenol triggers common carp hepatocytes ferroptosis via oxidative stress, iron overload, SLC7A11/GSH/GPX4 axis, and ATF4/HSPA5/GPX4 axis. Ecotoxicol. Environ. Saf. 2022, 242, 113944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, J.; Wang, Y.; Lin, X.; Teng, X.H.; Tang, Y. Complex molecular mechanism of ammonia-induced apoptosis in chicken peripheral blood lymphocytes: miR-27b-3p, heat shock proteins, immunosuppression, death receptor pathway, and mitochondrial pathway. Ecotoxicol Environ. Saf. 2022, 236, 113471. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liang, J.; Jiang, C.; Wu, D.; Huang, B.; Teng, X.; Tang, Y. Mitochondrion Participated in Effect Mechanism of Manganese Poisoning on Heat Shock Protein and Ultrastructure of Testes in Chickens. Biol Trace Elem. Res. 2022, 6. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. J. Proteom. 2019, 19, 1800311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, H.; Zong, N. Effects of rapamycin and mammalian target of rapamycin (mTOR) on immune regulation advance. J. Chin J. Cell. Mol. Immunol. 2018, 34, 954–958. [Google Scholar]
- Couso, I.; Evans, S.B.; Li, J.; Liu, Y.; Ma, F.F.; Diamond, S.; Allen, D.K.; Umen, J.G. Synergism between inositol polyphosphates and TOR kinase signaling in nutrient sensing, growth control, and lipid metabolism in Chlamydomonas. The Plant Cell. 2016, 28, 2026–2042. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Yu, Q. Role of mTOR Signaling in Female Reproduction. Front Endocrinol. 2019, 10, 692. [Google Scholar] [CrossRef]
- Condon, K.J.; Sabatini, D.M. Nutrient regulation of mTORC1 at a glance. J. Cell Sci. 2019, 13, 132. [Google Scholar] [CrossRef]
- Fu, W.; Hall, M.N. Regulation of mTORC2 Signaling. Genes 2020, 11, 1045. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chen, B.; Zhu, Q.; Xu, Z.; Ning, C.; Yin, H.; Wang, Y.; Zhao, X.; Fan, X.; Yang, M.; et al. Transcriptome analysis reveals differentially expressed genes associated with high rates of egg production in chicken hypothalamic-pituitary-ovarian axis. Sci. Rep. 2020, 10, 5976. [Google Scholar] [CrossRef] [Green Version]
- Hao, E.Y.; Wang, D.H.; Chen, Y.F.; Zhou, R.Y.; Chen, H.; Huang, R.L. The relationship between the mTOR signaling pathway and ovarian aging in peak-phase and late-phase laying hens. Poult Sci. 2021, 100, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Herta, A.C.; Lolicato, F.; Smitz, J.E.J. In vitro follicle culture in the context of IVF. Reproduction 2018, 156, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, M.; Menon, K.M. Luteinizing hormone/human chorionic gonadotropin-mediated activation of mTORC1 signaling is required for androgen synthesis by theca-interstitial cells. J. Mol. Endocrinol. 2012, 26, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, T.; Guo, Y.S.; Sun, T.; Li, H.; Zhang, X.Y.; Yin, H.; Cao, G.Y.; Yin, Y.X.; Wang, H.; et al. Oocytestage-specific effects of mTOR determine granulosa cell fate andoocyte quality in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 5326–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, N.; Levine, B.; Cuervo, A.; Klion, D. Autophagy flights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Bio. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Cao, P.; Song, W.; Li, Y.; Wang, T.; Qian, H.; Yan, C.; Yan, N. Structural basis for sterol sensing by Scap and Insig. Cell Rep. 2021, 35, 109299. [Google Scholar] [CrossRef]
- Magnuson, B.; Ekim, B.; Fingar, D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signaling networks. Biochem. J. 2012, 441, 1–21. [Google Scholar] [CrossRef]









| Items | Amount |
|---|---|
| Dietary composition | |
| Corn (%) | 61.0 |
| Soybean meal (%) | 25.0 |
| Wheat bran (%) | 3.0 |
| CaCO3 (%) | 8.0 |
| aPremix compound (%) | 3.0 |
| Nutrient levels | |
| ME (MJ/kg−1) | 11.30 |
| CP (%) | 16.0 |
| Met (%) | 0.38 |
| Met + Cys (%) | 0.62 |
| Lys (%) | 0.53 |
| Arg (%) | 0.66 |
| Thr (%) | 0.46 |
| Trp (%) | 0.14 |
| CF (%) | 2.9 |
| Ash (%) | 16.0 |
| Ca (%) | 3.50 |
| TP (%) | 0.60 |
| Gene | GenBank Accession | Primer Sequences (5′→3′) | Size (bp) |
|---|---|---|---|
| CYP11A1 | NM_001001756.2 | AGGGAGAAGTTGGGTGTCTACGA/ CTTGTTGCGGTAGTCACGGTAT | 138 |
| StAR | NM_204686.3 | CCATCTCCTACCAACACCTGC/ CGAGGATGCTGAGTGATTTCTG | 240 |
| CYP19A1 | NM_001001761.4 | TGCTGCTCCTGATACTCTGTCC/ AAGTCCACAACTGGCTGGTATCT | 201 |
| FSHR | NM_205079.2 | TTTTCCAGCCTTCCCAAACTAC/ ACCTTATGGACGACGGGTAAAA | 152 |
| SREBP1 | NM_204126.3 | AGTGGACCCGTTGGCTCA/ TGAAGGTACTCCAACGCATCC | 141 |
| S6K | NM_001012587.3 | TATGCCTTCCAGACAGATACAAAGC/ GACCAAAGTCTGTCAGCACCAC | 238 |
| GPX4 | NM_001346448.2 | AGGGGCTTCGTCTGCATCAT/ TCCTGCTTCCCGAACTGGTT | 149 |
| p53 | NM_205264.1 | ATCCTCACCATCCTTACACTGGA/ CCTCATTGATCTCCTTCAGCATCT | 280 |
| SLC7A11 | XM_426289.7 | TCCTGCTTTGGGTCTATGAATG/ ACATTATCATTGTGAGAGGGTGCA | 163 |
| β-action | NM_205518.1 | CTGACTGACCGCGTTACTCC/ TTGCACATACCGGAGCCATT | 84 |
| Genes | Primary Ab | Secondary Ab | Dilution Ratio | Resource |
|---|---|---|---|---|
| GPX4 | Anti-Rabbit | Peroxidase-Conjugated Goat Anti-Rabbit IgG | 1:500/1:200 | Wu han servicebio technology CO., LTD |
| p53 | Anti-Rabbit | Peroxidase-Conjugated Goat Anti-Rabbit IgG | 1:200/1:200 | Wu han servicebio technology CO., LTD |
| S6K | Anti-Rabbit | Peroxidase-Conjugated Goat Anti-Rabbit IgG | 1:500/1:200 | Wu han servicebio technology CO., LTD |
| SREBP1 | Anti-Rabbit | Peroxidase-Conjugated Goat Anti-Rabbit IgG | 1:1000/1:200 | Wu han servicebio technology CO., LTD |
| Items | Total a | Hits b | p-Value | −log10 (p Value) | Impact Value c | Metabolites |
|---|---|---|---|---|---|---|
| mTOR signaling pathway | 4 | 2 | 0.001 | 2.7633 | 0.500 | AMP; L-Arginine |
| Glutathione metabolism | 38 | 4 | 0.003 | 2.4177 | 0.3021 | Glutathione; Ornithine; Oxidized glutathione; Glutamylcysteine |
| Ferroptosis | 29 | 3 | 0.013 | 1.8854 | 0.2093 | Glutathione; Oxidized glutathione; γ-Glutamylcysteine |
| ABC transporters | 138 | 7 | 0.008 | 2.0553 | 0.0507 | Glutathione; L-Arginine; Ornithine; Mannitol; Sorbitol; 4-Hydroxyproline; Methyl beta-D-galactosidase |
| D-Arginine D-ornithine metabolism | 11 | 2 | 0.014 | 1.8349 | 0.1333 | L-Arginine; Ornithine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Pan, H.; Zhao, W.; Li, W.; Li, H.; Tian, Z.; Meng, D.; Teng, Y.; Li, X.; He, Y.; et al. Untargeted Metabolomics Revealed Potential Biomarkers of Small Yellow Follicles of Chickens during Sexual Maturation. Metabolites 2023, 13, 176. https://doi.org/10.3390/metabo13020176
Zhao J, Pan H, Zhao W, Li W, Li H, Tian Z, Meng D, Teng Y, Li X, He Y, et al. Untargeted Metabolomics Revealed Potential Biomarkers of Small Yellow Follicles of Chickens during Sexual Maturation. Metabolites. 2023; 13(2):176. https://doi.org/10.3390/metabo13020176
Chicago/Turabian StyleZhao, Jinbo, Hongbin Pan, Wenjiang Zhao, Wei Li, Haojie Li, Zhongxiao Tian, Dayong Meng, Yuting Teng, Xinlu Li, Yang He, and et al. 2023. "Untargeted Metabolomics Revealed Potential Biomarkers of Small Yellow Follicles of Chickens during Sexual Maturation" Metabolites 13, no. 2: 176. https://doi.org/10.3390/metabo13020176