Paper-Based Humidity Sensor for Respiratory Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensor Fabrication
2.2. Sensor Calibration Setup
2.3. Signal Acquisition System
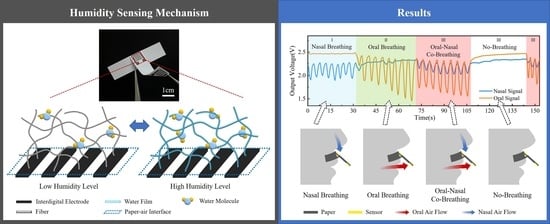
3. Results
3.1. Paper Humidity Sensing Model
3.2. Humidity Sensing Performance on the Paper-Based Humidity Sensor
3.3. Respiratory Monitoring of Simulated Breathing Signal
3.4. Sleep Respiratory Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comroe, J.H. Physiology of respiration. Acad. Med. 1965, 40, 887. [Google Scholar]
- Wallace, A.; Bucks, R.S. Memory and Obstructive Sleep Apnea: A Meta-Analysis. Sleep 2013, 36, 203–220. [Google Scholar] [CrossRef]
- Schumann, R.; Kwater, A.P.; Bonney, I.; Ladd, D.; Kim, J.; Gupta, A.; Gumbert, S.D.; Pivalizza, E.G. Respiratory volume monitoring in an obese surgical population and the prediction of postoperative respiratory depression by the STOP-bang OSA risk score. J. Clin. Anesth. 2016, 34, 295–301. [Google Scholar] [CrossRef]
- Corral-Peñafiel, J.; Pépin, J.L.; Barbé, F. Ambulatory monitoring in the diagnosis and management of obstructive sleep apnoea syndrome. Eur. Respir. Rev. 2013, 22, 312–324. [Google Scholar] [CrossRef]
- Pacheco, M.C.T.; Casagrande, C.F.; Teixeira, L.P.; Finck, N.S.; de Araújo, M.T.M. Guidelines proposal for clinical recognition of mouth breathing children. Dent. Press J. Orthod. 2015, 20, 39–44. [Google Scholar] [CrossRef]
- De Menezes, V.A.; Leal, R.B.; Pessoa, R.S.; Pontes, R.M.E.S. Prevalência e fatores associados à respiração oral em escolares participantes do projeto Santo Amaro-Recife, 2005. Rev. Bras. Otorrinolaringol. 2006, 72, 394–399. [Google Scholar] [CrossRef]
- Orr, W.C. Utilization of Polysomnography in the Assessment of Sleep Disorders. Med. Clin. N. Am. 1985, 69, 1153–1167. [Google Scholar] [CrossRef]
- Rundo, J.V.; Ralph, D., III. Polysomnography. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 381–392. [Google Scholar]
- Escobedo, P.; Fernández-Ramos, M.D.; López-Ruiz, N.; Moyano-Rodríguez, O.; Martínez-Olmos, A.; de Vargas-Sansalvador, I.M.P.; Carvajal, M.A.; Capitán-Vallvey, L.F.; Palma, A.J. Smart facemask for wireless CO2 monitoring. Nat. Commun. 2022, 13, 72. [Google Scholar] [CrossRef]
- Martin, A.; Voix, J. In-Ear Audio Wearable: Measurement of Heart and Breathing Rates for Health and Safety Monitoring. IEEE Trans. Biomed. Eng. 2017, 65, 1256–1263. [Google Scholar] [CrossRef]
- Li, B.; Tian, Q.; Su, H.; Wang, X.; Wang, T.; Zhang, D. High sensitivity portable capacitive humidity sensor based on In2O3 nanocubes-decorated GO nanosheets and its wearable application in respiration detection. Sens. Actuators B Chem. 2019, 299, 126973. [Google Scholar] [CrossRef]
- Manoni, A.; Loreti, F.; Radicioni, V.; Pellegrino, D.; Della Torre, L.; Gumiero, A.; Halicki, D.; Palange, P.; Irrera, F. A New Wearable System for Home Sleep Apnea Testing, Screening, and Classification. Sensors 2020, 20, 7014. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.; Kim, J.H.; Entifar, S.A.N.; Prameswati, A.; Wibowo, A.F.; Kim, S.; Lim, D.C.; Lee, J.; Moon, M.-W.; et al. Stretchable and Conductive Cellulose/Conductive Polymer Composite Films for On-Skin Strain Sensors. Materials 2022, 15, 5009. [Google Scholar] [CrossRef]
- Aliverti, A. Wearable technology: Role in respiratory health and disease. Breathe 2017, 13, e27–e36. [Google Scholar] [CrossRef]
- Koch, E.; Dietzel, A. Respiratory trigger signal generation by means of a stretchable sensor array. Sens. Actuators A Phys. 2018, 273, 113–120. [Google Scholar] [CrossRef]
- Massaroni, C.; Schena, E.; Silvestri, S.; Taffoni, F.; Merone, M. Measurement system based on RBG camera signal for contactless breathing pattern and respiratory rate monitoring. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018. [Google Scholar]
- Chen, Y.; Liu, F.; Lu, B.; Zhang, Y.; Feng, X. Skin-Like Hybrid Integrated Circuits Conformal to Face for Continuous Respiratory Monitoring. Adv. Electron. Mater. 2020, 6, 2000145. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J. Recent advancements in flexible humidity sensors. J. Semicond. 2020, 41, 040401. [Google Scholar] [CrossRef]
- Zhu, P.; Peng, H.; Rwei, A.Y. Flexible, wearable biosensors for digital health. Med. Nov. Technol. Devices 2022, 14, 100118. [Google Scholar] [CrossRef]
- Lin, X.; Gao, S.; Fei, T.; Liu, S.; Zhao, H.; Zhang, T. Study on a paper-based piezoresistive sensor applied to monitoring human physiological signals. Sens. Actuators A Phys. 2019, 292, 66–70. [Google Scholar] [CrossRef]
- de Medeiros, M.S.; Chanci, D.; Martinez, R.V. Moisture-insensitive, self-powered paper-based flexible electronics. Nano Energy 2020, 78, 105301. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Wang, H.; Han, Y.; Liu, J.; Lu, G.; Yu, H. A MXene-functionalized paper-based electrochemical immunosensor for label-free detection of cardiac troponin I. J. Semicond. 2021, 42, 092601. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Lee, N.Y. A rapid and eco-friendly isothermal amplification microdevice for multiplex detection of foodborne pathogens. Lab Chip 2018, 18, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.C.; Panicker, P.M.; Qiu, X.; Benjamin, A.J.; Quintana Soler, R.A.; Wils, I.; Hübler, A.C. Paper-embedded roll-to-roll mass printed piezoelectric transducers. Adv. Mater. 2021, 33, 2006437. [Google Scholar] [CrossRef] [PubMed]
- Jürgen, W.; Stocker, D.; Dörsam, E. Characteristics and evaluation criteria of substrate-based manufacturing. Is roll-to-roll the best solution for printed electronics? Org. Electron. 2014, 15, 1631–1640. [Google Scholar]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2010, 136, 77–82. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.; Martínez-Paredes, G.; Añorga, L.; Grande, H. Glucose biosensor based on disposable electrochemical paper-based transducers fully fabricated by screen-printing. Biosens. Bioelectron. 2018, 109, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Mezic, I. Instability of Ultra-Thin Water Films and the Mechanism of Droplet Formation on Hydrophilic Surfaces. J. Heat Transf. 1999, 121, 964–971. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Mansour, E.; Vishinkin, R.; Rihet, S.; Saliba, W.; Fish, F.; Sarfati, P.; Haick, H. Measurement of temperature and relative humidity in exhaled breath. Sens. Actuators B Chem. 2019, 304, 127371. [Google Scholar] [CrossRef]
- Hartley, J. Respiratory rate 2: Anatomy and physiology of breathing. Nurs. Times 2018, 104, 43–44. [Google Scholar]
- Edouard, P.; Campo, D.; Bartet, P.; Yang, R.Y.; Bruyneel, M.; Roisman, G.; Escourrou, P. Validation of the Withings Sleep Analyzer, an under-the-mattress device for the detection of moderate-severe sleep apnea syndrome. J. Clin. Sleep Med. 2021, 17, 1217–1227. [Google Scholar] [CrossRef]
- Asadi, A.; Niebuhr, O.; Jørgensen, J.; Fischer, K. Inducing Changes in Breathing Patterns Using a Soft Robot. In Proceedings of the 2022 ACM/IEEE International Conference on Human-Robot Interaction, online, 7–10 March 2022. [Google Scholar]
- Lu, Q.; Chen, H.; Zeng, Y.; Xue, J.; Cao, X.; Wang, N.; Wang, Z. Intelligent facemask based on triboelectric nanogenerator for respiratory monitoring. Nano Energy 2021, 91, 106612. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Pang, B.; Li, P.; Yao, Z.; Zhang, X.; Chen, H. A new physiological signal acquisition patch designed with advanced respiration monitoring algorithm based on 3-axis accelerator and gyroscope. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Piscataway, NJ, USA, 2018. [Google Scholar]
- Zhang, H.; Zhang, P.; Li, Y.; Zheng, R.; Ding, G.; Xiao, Q.; Su, K.; Yin, S.; Yang, Z. A Wearable Ultrathin Flexible Sensor Inserted Into Nasal Cavity for Precise Sleep Respiratory Monitoring. IEEE Trans. Electron Devices 2021, 68, 4090–4097. [Google Scholar] [CrossRef]
- Massaroni, C.; Di Tocco, J.; Presti, D.L.; Longo, U.G.; Miccinilli, S.; Sterzi, S.; Formica, D.; Saccomandi, P.; Schena, E. Smart Textile Based on Piezoresistive Sensing Elements for Respiratory Monitoring. IEEE Sens. J. 2019, 19, 7718–7725. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, S.; Zou, P.; Li, R.; Fan, Y. Paper-Based Humidity Sensor for Respiratory Monitoring. Materials 2022, 15, 6447. https://doi.org/10.3390/ma15186447
Ma X, Zhang S, Zou P, Li R, Fan Y. Paper-Based Humidity Sensor for Respiratory Monitoring. Materials. 2022; 15(18):6447. https://doi.org/10.3390/ma15186447
Chicago/Turabian StyleMa, Xiaoxiao, Shaoxing Zhang, Peikai Zou, Ruya Li, and Yubo Fan. 2022. "Paper-Based Humidity Sensor for Respiratory Monitoring" Materials 15, no. 18: 6447. https://doi.org/10.3390/ma15186447